
Galvani potential
Encyclopedia
Galvani potential in electrochemistry
, is the electric potential difference between two points in the bulk of two phases. These phases can be two different solids (e.g., two metals joint together), or a solid and a liquid (e.g., a metal electrode
submerged in an electrolyte
).
Generally, the Galvani potential difference is measurable only when the two phases have identical chemical composition.
The Galvani potential is named after Luigi Galvani
.
to the metal with the higher work function until the electrochemical potential of the electrons in the bulk of both phases are equal. The actual numbers of electrons that passes between the two phases is small, and the occupancy of the Fermi level
s is practically unaffected. Rather, this small increase or decrease in charge results in a shift in all the energy levels in the metals. An electrical double layer
is formed at the interface between the two phases.
The equality of the electrochemical potential between the two different phases in contact can be written as:
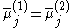
where:
Now, the electrochemical potential of a species is defined as a sum of its chemical potential and the local electrostatic potential:
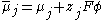
where:
From the two equations above: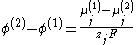
where the difference on the left-hand side is the Galvani potential difference between the phases (1) and (2). Thus, the Galvani potential difference is determined entirely by chemical identity of the two phases; specifically by the difference of the chemical potential of the charge carriers in the two phases.
The Galvani potential difference between an electrode and electrolyte (or between other two electrically conductive phases) forms in an analogous fashion, although the chemical potentials in the equation above may need to include all species involved in the electrochemical reaction at the interface.
:
where:
Instead, the measured cell potential can be written as:
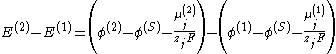
where:
From the above equation, two metals in electronic contact (i.e., under electronic equilibrium) must have the same electrode potential. Also, the electrochemical potentials of the electrons within the two metals will be the same. However, their Galvani potentials will be different (unless the metals are identical).
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
, is the electric potential difference between two points in the bulk of two phases. These phases can be two different solids (e.g., two metals joint together), or a solid and a liquid (e.g., a metal electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
submerged in an electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
).
Generally, the Galvani potential difference is measurable only when the two phases have identical chemical composition.
The Galvani potential is named after Luigi Galvani
Luigi Galvani
Luigi Aloisio Galvani was an Italian physician and physicist who lived and died in Bologna. In 1791, he discovered that the muscles of dead frogs legs twitched when struck by a spark...
.
Galvani potential between two metals
First, consider the Galvani potential between two metals. When two metals are electrically isolated from each other, an arbitrary potential difference may exist between them. However, when two different metals are brought into electronic contact, electrons will flow from the metal with a lower work functionWork function
In solid-state physics, the work function is the minimum energy needed to remove an electron from a solid to a point immediately outside the solid surface...
to the metal with the higher work function until the electrochemical potential of the electrons in the bulk of both phases are equal. The actual numbers of electrons that passes between the two phases is small, and the occupancy of the Fermi level
Fermi level
The Fermi level is a hypothetical level of potential energy for an electron inside a crystalline solid. Occupying such a level would give an electron a potential energy \epsilon equal to its chemical potential \mu as they both appear in the Fermi-Dirac distribution function,which...
s is practically unaffected. Rather, this small increase or decrease in charge results in a shift in all the energy levels in the metals. An electrical double layer
Double layer (interfacial)
A double layer is a structure that appears on the surface of an object when it is placed into a liquid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object...
is formed at the interface between the two phases.
The equality of the electrochemical potential between the two different phases in contact can be written as:
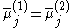
where:
-
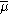 is the electrochemical potential
is the electrochemical potential - j denotes the species which are the carrier of electrical current in the system (which are electrons in metals)
- (1) and (2) denote phase 1 and phase 2, respectively.
Now, the electrochemical potential of a species is defined as a sum of its chemical potential and the local electrostatic potential:
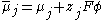
where:
- μ is the chemical potentialChemical potentialChemical potential, symbolized by μ, is a measure first described by the American engineer, chemist and mathematical physicist Josiah Willard Gibbs. It is the potential that a substance has to produce in order to alter a system...
- z is the electrical charge carried by a single charge carrier (unity for electrons)
- F is the Faraday constant
- Φ is the electrostatic potential
From the two equations above:
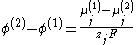
where the difference on the left-hand side is the Galvani potential difference between the phases (1) and (2). Thus, the Galvani potential difference is determined entirely by chemical identity of the two phases; specifically by the difference of the chemical potential of the charge carriers in the two phases.
The Galvani potential difference between an electrode and electrolyte (or between other two electrically conductive phases) forms in an analogous fashion, although the chemical potentials in the equation above may need to include all species involved in the electrochemical reaction at the interface.
Relation to measured cell potential
The Galvani potential difference is not measurable. The measured potential difference between two metal electrodes assembled into a cell does not equal the difference of the Galvani potentials of the two metals (or their combination with the solution Galvani potential) because the cell needs to contains another metal-metal interface, as in the following schematic of a galvanic cellGalvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
:
- M(1) | S | M(2) | M(1)'
where:
- M(1) and M(2) are the two different metals,
- S denotes the electrolyte,
- M(1)' is the additional metal (here assumed to be the metal (1)) that must be inserted into the circuit to close it,
- the vertical bar, |, denotes a phase boundary.
Instead, the measured cell potential can be written as:
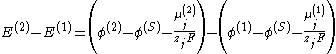
where:
- E is the potential of a single electrode,
- (S) denotes the electrolyte solution.
From the above equation, two metals in electronic contact (i.e., under electronic equilibrium) must have the same electrode potential. Also, the electrochemical potentials of the electrons within the two metals will be the same. However, their Galvani potentials will be different (unless the metals are identical).

