Work function
Encyclopedia
In solid-state physics
, the work function is the minimum energy
(usually measured in electron volts) needed to remove an electron
from a solid to a point immediately outside the solid surface (or energy needed to move an electron from the Fermi level
into vacuum). Here "immediately" means that the final electron position is far from the surface on the atomic scale but still close to the solid on the macroscopic scale. The work function is a characteristic property for any solid face of a substance with a conduction band
(whether empty or partly filled). For a metal, the Fermi level is inside the conduction band, indicating that the band is partly filled. For an insulator, the Fermi level lies within the band gap, indicating an empty conduction band; in this case, the minimum energy to remove an electron is about the sum of half the band gap and the electron affinity.
, electron excitation
is achieved by absorption of a photon
. If the photon's energy is greater than the substance's work function, photoelectric emission occurs and the electron is liberated from the surface. Excess photon energy results in a liberated electron with non-zero kinetic energy. The photoelectric work function is: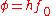
where is Planck's constant and
is Planck's constant and 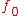 is the minimum (threshold) frequency of the photon required to produce photoelectric emission.
is the minimum (threshold) frequency of the photon required to produce photoelectric emission.
. Here the electron gains its energy from heat rather than photons. According to the Richardson-Dushman equation
the emitted electron current density
, J (A/m2), is related to the absolute temperature
T by the equation:
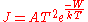
where W is the work function of the metal, k is the Boltzmann constant and the proportionality constant A, known as Richardson's constant, is given by

where m and -e are the mass and charge of an electron, and h is Planck's constant.
Thermionic emission—electrons escaping from the heated negatively-charged filament (hot cathode
)—is important in the operation of vacuum tubes.
Tungsten
, the common choice for vacuum tube filaments, has a work function of approximately 4.5 eV. various
oxide coatings can substantially reduce this.
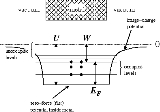
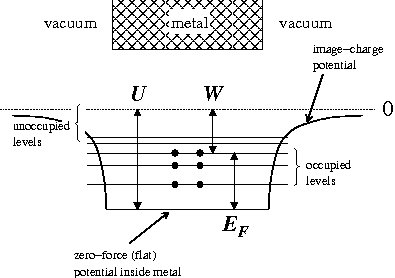 In the free electron model
In the free electron model
the valence electrons roam freely (zero force) inside the metal but find
a confining potential step at the boundary of the metal. In the system's ground state, states
at the boundary of the metal. In the system's ground state, states
with energy less than the Fermi Level are occupied, and states above the Fermi Level are not occupied. The energy
required to liberate an electron in the Fermi Level is the work function. If, as in the diagram right, we define
the Fermi Energy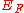 from the bottom of the well, the results reported in the Wiki page Fermi Energy
from the bottom of the well, the results reported in the Wiki page Fermi Energy
are applicable. However, usually the Fermi Energy is referenced to energy zero: that of the lowest energy electron
free of the metal. In that case the Fermi Energy would have a negative value (i.e., the Fermi Level lies
below those of escaped electrons)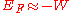 (but see below).
(but see below).
The magnitude of the work function is usually about a half of the ionization energy
of a free atom of the metal.
For example, caesium
has ionization energy 3.9 eV and work function 2.14 eV.
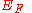 (defined
(defined
relative to the lowest energy free particle: zero in the above diagram) yet the two quantities are not exactly the same. This is due to the surface effect of a real-world solid: a real-world solid is not infinitely extended with electrons and ions repeatedly filling every primitive cell
over all Bravais lattice sites. Neither can one simply take a set of Bravais lattice sites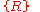 inside the geometrical region V which the solid occupies and then fill undistorted charge distribution basis into all primitive cells of
inside the geometrical region V which the solid occupies and then fill undistorted charge distribution basis into all primitive cells of  . Indeed, the charge distribution in those cells near the surface will be distorted significantly from that in a cell of an ideal infinite solid, resulting in an effective surface dipole distribution, or, sometimes both a surface dipole distribution and a surface charge distribution.
. Indeed, the charge distribution in those cells near the surface will be distorted significantly from that in a cell of an ideal infinite solid, resulting in an effective surface dipole distribution, or, sometimes both a surface dipole distribution and a surface charge distribution.
It can be proven that if we define work function as the minimum energy needed to remove an electron to a point immediately out of the solid, the effect of the surface charge distribution can be neglected, leaving only the surface dipole distribution. Let the potential energy difference across the surface due to effective surface dipole be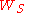 . And let
. And let 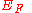 be the Fermi energy
be the Fermi energy
calculated for the finite solid without considering surface distortion effect, when taking the convention that the potential at is zero. Then, the correct formula for work function is:
is zero. Then, the correct formula for work function is:
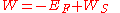
Where is negative, which means that electrons are bound in the solid.
is negative, which means that electrons are bound in the solid.
the work function is important for design of the metal-semiconductor junction
in Schottky diode
s and for design of vacuum tube
s. The work function difference between metal and silicon in a MOS capacitor is related to the flat-band voltage (i.e. the voltage that induces zero net charge in the underlying semiconductor) and the equivalent oxide charge per unit area at the oxide-silicon interface by
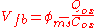 .
.
Methods of the first group employ electron emission from the sample induced by photon absorption (photoemission), by high temperature (thermionic emission), due to an electric field (field electron emission), or using electron tunnelling.
All relative methods make use of the contact potential difference between the sample and a reference electrode. Experimentally, either an anode current of a diode is used or the displacement current between the sample and reference, created by an artificial change in the capacitance between the two, is measured (the Kelvin Probe method, Kelvin probe force microscope
).
(UV) light and the kinetic energy
of the emitted electrons is analysed. As UV light is electromagnetic radiation
with an energy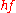 lower than 100 eV it is able to extract mainly valence electrons. Due to limitations of the escape depth of electrons in solids, UPS is very surface sensitive, as the information depth is in the range of 2 – 20 monolayers (1-10 nm). The resulting spectrum reflects the electronic structure of the sample providing information on the density of states, the occupation of states, and the work function.
lower than 100 eV it is able to extract mainly valence electrons. Due to limitations of the escape depth of electrons in solids, UPS is very surface sensitive, as the information depth is in the range of 2 – 20 monolayers (1-10 nm). The resulting spectrum reflects the electronic structure of the sample providing information on the density of states, the occupation of states, and the work function.
method is one of the simplest and oldest method of measuring work functions. It is based on the thermionic emission of electrons from an emitter. The current density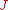 of the electrons collected by the sample depends on the work function
of the electrons collected by the sample depends on the work function  of the sample and is given by the Richardson–Dushman equation
of the sample and is given by the Richardson–Dushman equation 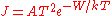 where
where  , the Richardson constant, is a specific material constant. The current density increases rapidly with temperature and decreases exponentially with the work function. Changes of the work function can be easily determined by applying a retarding potential
, the Richardson constant, is a specific material constant. The current density increases rapidly with temperature and decreases exponentially with the work function. Changes of the work function can be easily determined by applying a retarding potential  between the sample and the electron emitter;
between the sample and the electron emitter;  is replaced by
is replaced by  in above equation. The difference in the retarding potential measured at constant current is equivalent to the work function change, assuming that the work function and the temperature of the emitter is constant.
in above equation. The difference in the retarding potential measured at constant current is equivalent to the work function change, assuming that the work function and the temperature of the emitter is constant.
One can use the Richardson–Dushman equation directly to determine the work function by temperature variation of the sample, as well. Rearranging the equation yields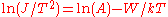 . The line produced by plotting
. The line produced by plotting 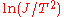 vs.
vs. 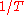 will have a slope of
will have a slope of 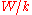 allowing to determine the work function of the sample.
allowing to determine the work function of the sample.
reference: CRC handbook on Chemistry and Physics version 2008, p. 12-114.
Note: Work function can change for crystalline elements based upon the orientation. For example Ag:4.26, Ag(100):4.64, Ag(110):4.52, Ag(111):4.74. Ranges for typical surfaces are shown in the table below.
Solid-state physics
Solid-state physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from...
, the work function is the minimum energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
(usually measured in electron volts) needed to remove an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
from a solid to a point immediately outside the solid surface (or energy needed to move an electron from the Fermi level
Fermi level
The Fermi level is a hypothetical level of potential energy for an electron inside a crystalline solid. Occupying such a level would give an electron a potential energy \epsilon equal to its chemical potential \mu as they both appear in the Fermi-Dirac distribution function,which...
into vacuum). Here "immediately" means that the final electron position is far from the surface on the atomic scale but still close to the solid on the macroscopic scale. The work function is a characteristic property for any solid face of a substance with a conduction band
Conduction band
In the solid-state physics field of semiconductors and insulators, the conduction band is the range of electron energies, higher than that of the valence band, sufficient to free an electron from binding with its individual atom and allow it to move freely within the atomic lattice of the material...
(whether empty or partly filled). For a metal, the Fermi level is inside the conduction band, indicating that the band is partly filled. For an insulator, the Fermi level lies within the band gap, indicating an empty conduction band; in this case, the minimum energy to remove an electron is about the sum of half the band gap and the electron affinity.
Photoelectric work function
The work function is the minimum energy that must be given to an electron to liberate it from the surface of a particular substance. In the photoelectric effectPhotoelectric effect
In the photoelectric effect, electrons are emitted from matter as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as photoelectrons...
, electron excitation
Electron excitation
Electron excitation is the movement of an electron to a higher energy state. This can either be done by photoexcitation , where the original electron absorbs the photon and gains all the photon's energy or by electrical excitation , where the original electron absorbs the energy of another,...
is achieved by absorption of a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
. If the photon's energy is greater than the substance's work function, photoelectric emission occurs and the electron is liberated from the surface. Excess photon energy results in a liberated electron with non-zero kinetic energy. The photoelectric work function is:
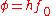
where
 is Planck's constant and
is Planck's constant and 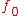 is the minimum (threshold) frequency of the photon required to produce photoelectric emission.
is the minimum (threshold) frequency of the photon required to produce photoelectric emission.Thermionic work function
The work function is also important in the theory of thermionic emissionThermionic emission
Thermionic emission is the heat-induced flow of charge carriers from a surface or over a potential-energy barrier. This occurs because the thermal energy given to the carrier overcomes the binding potential, also known as work function of the metal. The charge carriers can be electrons or ions, and...
. Here the electron gains its energy from heat rather than photons. According to the Richardson-Dushman equation
the emitted electron current density
Current density
Current density is a measure of the density of flow of a conserved charge. Usually the charge is the electric charge, in which case the associated current density is the electric current per unit area of cross section, but the term current density can also be applied to other conserved...
, J (A/m2), is related to the absolute temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
T by the equation:
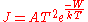
where W is the work function of the metal, k is the Boltzmann constant and the proportionality constant A, known as Richardson's constant, is given by

where m and -e are the mass and charge of an electron, and h is Planck's constant.
Thermionic emission—electrons escaping from the heated negatively-charged filament (hot cathode
Hot cathode
In vacuum tubes, a hot cathode is a cathode electrode which emits electrons due to thermionic emission. In the accelerator community, these are referred to as thermionic cathodes. The heating element is usually an electrical filament...
)—is important in the operation of vacuum tubes.
Tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
, the common choice for vacuum tube filaments, has a work function of approximately 4.5 eV. various
oxide coatings can substantially reduce this.
Free Electron Gas Model

Free electron model
In solid-state physics, the free electron model is a simple model for the behaviour of valence electrons in a crystal structure of a metallic solid. It was developed principally by Arnold Sommerfeld who combined the classical Drude model with quantum mechanical Fermi-Dirac statistics and hence it...
the valence electrons roam freely (zero force) inside the metal but find
a confining potential step
 at the boundary of the metal. In the system's ground state, states
at the boundary of the metal. In the system's ground state, stateswith energy less than the Fermi Level are occupied, and states above the Fermi Level are not occupied. The energy
required to liberate an electron in the Fermi Level is the work function. If, as in the diagram right, we define
the Fermi Energy
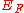 from the bottom of the well, the results reported in the Wiki page Fermi Energy
from the bottom of the well, the results reported in the Wiki page Fermi EnergyFermi energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy of the highest occupied quantum state in a system of fermions at absolute zero temperature....
are applicable. However, usually the Fermi Energy is referenced to energy zero: that of the lowest energy electron
free of the metal. In that case the Fermi Energy would have a negative value (i.e., the Fermi Level lies
below those of escaped electrons)
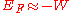 (but see below).
(but see below).Work Function Trends
The thermionic work function depends on the orientation of the crystal and will tend to be smaller for metals with an open lattice, larger for metals in which the atoms are closely packed. The range is about 1.5–6 eV. It is somewhat higher on dense crystal faces than open ones.The magnitude of the work function is usually about a half of the ionization energy
Ionization energy
The ionization energy of a chemical species, i.e. an atom or molecule, is the energy required to remove an electron from the species to a practically infinite distance. Large atoms or molecules have a low ionization energy, while small molecules tend to have higher ionization energies.The property...
of a free atom of the metal.
For example, caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
has ionization energy 3.9 eV and work function 2.14 eV.
Work Function and Surface Effect
The work function W of a metal is closely related to its Fermi energyFermi energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy of the highest occupied quantum state in a system of fermions at absolute zero temperature....
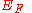 (defined
(definedrelative to the lowest energy free particle: zero in the above diagram) yet the two quantities are not exactly the same. This is due to the surface effect of a real-world solid: a real-world solid is not infinitely extended with electrons and ions repeatedly filling every primitive cell
Primitive cell
Used predominantly in geometry, solid state physics, and mineralogy, particularly in describing crystal structure, a primitive cell is a minimum cell corresponding to a single lattice point of a structure with translational symmetry in 2 dimensions, 3 dimensions, or other dimensions...
over all Bravais lattice sites. Neither can one simply take a set of Bravais lattice sites
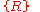 inside the geometrical region V which the solid occupies and then fill undistorted charge distribution basis into all primitive cells of
inside the geometrical region V which the solid occupies and then fill undistorted charge distribution basis into all primitive cells of  . Indeed, the charge distribution in those cells near the surface will be distorted significantly from that in a cell of an ideal infinite solid, resulting in an effective surface dipole distribution, or, sometimes both a surface dipole distribution and a surface charge distribution.
. Indeed, the charge distribution in those cells near the surface will be distorted significantly from that in a cell of an ideal infinite solid, resulting in an effective surface dipole distribution, or, sometimes both a surface dipole distribution and a surface charge distribution.It can be proven that if we define work function as the minimum energy needed to remove an electron to a point immediately out of the solid, the effect of the surface charge distribution can be neglected, leaving only the surface dipole distribution. Let the potential energy difference across the surface due to effective surface dipole be
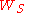 . And let
. And let 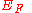 be the Fermi energy
be the Fermi energyFermi energy
The Fermi energy is a concept in quantum mechanics usually referring to the energy of the highest occupied quantum state in a system of fermions at absolute zero temperature....
calculated for the finite solid without considering surface distortion effect, when taking the convention that the potential at
 is zero. Then, the correct formula for work function is:
is zero. Then, the correct formula for work function is: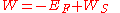
Where
 is negative, which means that electrons are bound in the solid.
is negative, which means that electrons are bound in the solid.Applications
In electronicsElectronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
the work function is important for design of the metal-semiconductor junction
Metal-semiconductor junction
In solid-state physics, a metal–semiconductor junction is a type of junction in which a metal comes in close contact with a semiconductor material...
in Schottky diode
Schottky diode
The Schottky diode is a semiconductor diode with a low forward voltage drop and a very fast switching action...
s and for design of vacuum tube
Vacuum tube
In electronics, a vacuum tube, electron tube , or thermionic valve , reduced to simply "tube" or "valve" in everyday parlance, is a device that relies on the flow of electric current through a vacuum...
s. The work function difference between metal and silicon in a MOS capacitor is related to the flat-band voltage (i.e. the voltage that induces zero net charge in the underlying semiconductor) and the equivalent oxide charge per unit area at the oxide-silicon interface by
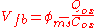 .
.Measurement
Many techniques have been developed based on different physical effects to measure the electronic work function of a sample. One may distinguish between two groups of experimental methods for work function measurements: absolute and relative.Methods of the first group employ electron emission from the sample induced by photon absorption (photoemission), by high temperature (thermionic emission), due to an electric field (field electron emission), or using electron tunnelling.
All relative methods make use of the contact potential difference between the sample and a reference electrode. Experimentally, either an anode current of a diode is used or the displacement current between the sample and reference, created by an artificial change in the capacitance between the two, is measured (the Kelvin Probe method, Kelvin probe force microscope
Kelvin probe force microscope
Kelvin probe force microscopy , also known as surface potential microscopy, is a noncontact variant of atomic force microscopy that was invented in 1991. With KPFM, the work function of surfaces can be observed at atomic or molecular scales...
).
Methods Based on Photoemission
Photoelectron emission spectroscopy (PES) is the general term for spectroscopic techniques based on the outer photoelectric effect. In the case of Ultraviolet Photoelectron Spectroscopy (UPS), the surface of a solid sample is irradiated with ultravioletUltraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
(UV) light and the kinetic energy
Kinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
of the emitted electrons is analysed. As UV light is electromagnetic radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
with an energy
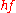 lower than 100 eV it is able to extract mainly valence electrons. Due to limitations of the escape depth of electrons in solids, UPS is very surface sensitive, as the information depth is in the range of 2 – 20 monolayers (1-10 nm). The resulting spectrum reflects the electronic structure of the sample providing information on the density of states, the occupation of states, and the work function.
lower than 100 eV it is able to extract mainly valence electrons. Due to limitations of the escape depth of electrons in solids, UPS is very surface sensitive, as the information depth is in the range of 2 – 20 monolayers (1-10 nm). The resulting spectrum reflects the electronic structure of the sample providing information on the density of states, the occupation of states, and the work function.Methods Based on Thermionic Emission
The retarding diodeDiode
In electronics, a diode is a type of two-terminal electronic component with a nonlinear current–voltage characteristic. A semiconductor diode, the most common type today, is a crystalline piece of semiconductor material connected to two electrical terminals...
method is one of the simplest and oldest method of measuring work functions. It is based on the thermionic emission of electrons from an emitter. The current density
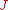 of the electrons collected by the sample depends on the work function
of the electrons collected by the sample depends on the work function  of the sample and is given by the Richardson–Dushman equation
of the sample and is given by the Richardson–Dushman equation 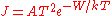 where
where  , the Richardson constant, is a specific material constant. The current density increases rapidly with temperature and decreases exponentially with the work function. Changes of the work function can be easily determined by applying a retarding potential
, the Richardson constant, is a specific material constant. The current density increases rapidly with temperature and decreases exponentially with the work function. Changes of the work function can be easily determined by applying a retarding potential  between the sample and the electron emitter;
between the sample and the electron emitter;  is replaced by
is replaced by  in above equation. The difference in the retarding potential measured at constant current is equivalent to the work function change, assuming that the work function and the temperature of the emitter is constant.
in above equation. The difference in the retarding potential measured at constant current is equivalent to the work function change, assuming that the work function and the temperature of the emitter is constant.One can use the Richardson–Dushman equation directly to determine the work function by temperature variation of the sample, as well. Rearranging the equation yields
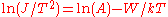 . The line produced by plotting
. The line produced by plotting 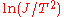 vs.
vs. 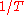 will have a slope of
will have a slope of 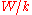 allowing to determine the work function of the sample.
allowing to determine the work function of the sample.Electron Work Functions of Elements
Units: eV electron Voltsreference: CRC handbook on Chemistry and Physics version 2008, p. 12-114.
Note: Work function can change for crystalline elements based upon the orientation. For example Ag:4.26, Ag(100):4.64, Ag(110):4.52, Ag(111):4.74. Ranges for typical surfaces are shown in the table below.
| Element | eV | Element | eV | Element | eV | Element | eV | Element | eV |
|---|---|---|---|---|---|---|---|---|---|
| Ag Silver Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal... : |
4.52-4.74 | Al Aluminium Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances.... : |
4.06-4.26 | As Arsenic Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid... : |
3.75 | Au Gold Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a... : |
5.1-5.47 | B Boron Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the... : |
~4.45 |
| Ba Barium Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with... : |
2.52-2.7 | Be Beryllium Beryllium is the chemical element with the symbol Be and atomic number 4. It is a divalent element which occurs naturally only in combination with other elements in minerals. Notable gemstones which contain beryllium include beryl and chrysoberyl... : |
4.98 | Bi Bismuth Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead... : |
4.34 | C Carbon Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds... : |
~5 | Ca Calcium Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust... : |
2.87 |
| Cd Cadmium Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low... : |
4.08 | Ce Cerium Cerium is a chemical element with the symbol Ce and atomic number 58. It is a soft, silvery, ductile metal which easily oxidizes in air. Cerium was named after the dwarf planet . Cerium is the most abundant of the rare earth elements, making up about 0.0046% of the Earth's crust by weight... : |
2.9 | Co Cobalt Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.... : |
5 | Cr Chromium Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable... : |
4.5 | Cs Caesium Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature... : |
2.14 |
| Cu Copper Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish... : |
4.53-5.10 | Eu Europium Europium is a chemical element with the symbol Eu and atomic number 63. It is named after the continent of Europe. It is a moderately hard silvery metal which readily oxidizes in air and water... : |
2.5 | Fe Iron Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust... : |
4.67-4.81 | Ga Gallium Gallium is a chemical element that has the symbol Ga and atomic number 31. Elemental gallium does not occur in nature, but as the gallium salt in trace amounts in bauxite and zinc ores. A soft silvery metallic poor metal, elemental gallium is a brittle solid at low temperatures. As it liquefies... : |
4.32 | Gd Gadolinium Gadolinium is a chemical element with the symbol Gd and atomic number 64. It is a silvery-white, malleable and ductile rare-earth metal. It is found in nature only in combined form. Gadolinium was first detected spectroscopically in 1880 by de Marignac who separated its oxide and is credited with... : |
2.90 |
| Hf Hafnium Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869. Hafnium was the penultimate stable... : |
3.9 | Hg Mercury (element) Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum... : |
4.475 | In Indium Indium is a chemical element with the symbol In and atomic number 49. This rare, very soft, malleable and easily fusible post-transition metal is chemically similar to gallium and thallium, and shows the intermediate properties between these two... : |
4.09 | Ir Iridium Iridium is the chemical element with atomic number 77, and is represented by the symbol Ir. A very hard, brittle, silvery-white transition metal of the platinum family, iridium is the second-densest element and is the most corrosion-resistant metal, even at temperatures as high as 2000 °C... : |
5.00-5.67 | K Potassium Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are... : |
2.29 |
| La Lanthanum Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and... : |
4 | Li Lithium Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly... : |
2.93 | Lu: | ~3.3 | Mg Magnesium Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole... : |
3.66 | Mn Manganese Manganese is a chemical element, designated by the symbol Mn. It has the atomic number 25. It is found as a free element in nature , and in many minerals... : |
4.1 |
| Mo Molybdenum Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores... : |
4.36-4.95 | Na Sodium Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride... : |
2.36 | Nb Niobium Niobium or columbium , is a chemical element with the symbol Nb and atomic number 41. It's a soft, grey, ductile transition metal, which is often found in the pyrochlore mineral, the main commercial source for niobium, and columbite... : |
3.95-4.87 | Nd: | 3.2 | Ni Nickel Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile... : |
5.04-5.35 |
| Os Osmium Osmium is a chemical element with the symbol Os and atomic number 76. Osmium is a hard, brittle, blue-gray or blue-blacktransition metal in the platinum family, and is the densest natural element. Osmium is twice as dense as lead. The density of osmium is , slightly greater than that of iridium,... : |
5.93 | Pb Lead Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed... : |
4.25 | Pd Palladium Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired... : |
5.22-5.6 | Pt Platinum Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal... : |
5.12-5.93 | Rb Rubidium Rubidium is a chemical element with the symbol Rb and atomic number 37. Rubidium is a soft, silvery-white metallic element of the alkali metal group. Its atomic mass is 85.4678. Elemental rubidium is highly reactive, with properties similar to those of other elements in group 1, such as very rapid... : |
2.261 |
| Re Rhenium Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has... : |
4.72 | Rh Rhodium Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed... : |
4.98 | Ru Ruthenium Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element... : |
4.71 | Sb Antimony Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite... : |
4.55-4.7 | Sc Scandium Scandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic transition metal, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids... : |
3.5 |
| Se Selenium Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium... : |
5.9 | Si Silicon Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table... : |
4.60-4.85 | Sm Samarium Samarium is a chemical element with the symbol Sm, atomic number 62 and atomic weight 150.36. It is a moderately hard silvery metal which readily oxidizes in air. Being a typical member of the lanthanide series, samarium usually assumes the oxidation state +3... : |
2.7 | Sn Tin Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4... : |
4.42 | Sr Strontium Strontium is a chemical element with the symbol Sr and the atomic number 38. An alkaline earth metal, strontium is a soft silver-white or yellowish metallic element that is highly reactive chemically. The metal turns yellow when exposed to air. It occurs naturally in the minerals celestine and... : |
~2.59 |
| Ta Tantalum Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory... : |
4.00-4.80 | Tb Terbium Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white rare earth metal that is malleable, ductile and soft enough to be cut with a knife... : |
3.00 | Te: | 4.95 | Th Thorium Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder.... : |
3.4 | Ti Titanium Titanium is a chemical element with the symbol Ti and atomic number 22. It has a low density and is a strong, lustrous, corrosion-resistant transition metal with a silver color.... : |
4.33 |
| Tl Thallium Thallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy... : |
~3.84 | U Uranium Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons... : |
3.63-3.90 | V Vanadium Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery gray, ductile and malleable transition metal. The formation of an oxide layer stabilizes the metal against oxidation. The element is found only in chemically combined form in nature... : |
4.3 | W Tungsten Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as... : |
4.32-5.22 | Y Yttrium Yttrium is a chemical element with symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and it has often been classified as a "rare earth element". Yttrium is almost always found combined with the lanthanides in rare earth minerals and is... : |
3.1 |
| Yb Ytterbium Ytterbium is a chemical element with the symbol Yb and atomic number 70. A soft silvery metallic element, ytterbium is a rare earth element of the lanthanide series and is found in the minerals gadolinite, monazite, and xenotime. The element is sometimes associated with yttrium or other related... : |
2.60 | Zn Zinc Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2... : |
3.63-4.9 | Zr Zirconium Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium... : |
4.05 | ||||

