Favorskii rearrangement
Encyclopedia
The Favorskii rearrangement (not to be confused with the Favorskii reaction
), named for the Russian chemist Alexei Yevgrafovich Favorskii
, is most principally a rearrangement of cyclopropanone
s and α-halo ketones which leads to carboxylic acid
derivatives. In the case of cyclic α-halo ketones, the Favorski rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide
, to yield a carboxylic acid but most of the time either an alkoxide
base or an amine
to yield an ester
or an amide
, respectively. α,α’-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.
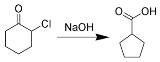
In the case of cylic α-halo ketones, the rearrangement occurs as depicted below :
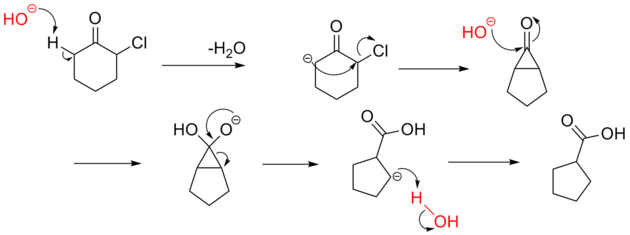
is thought to involve the formation of an enolate on the side of the ketone
away from the chlorine
atom. This enolate cyclizes to a cyclopropanone
intermediate which is then attacked by the hydroxide nucleophile
.
 Usage of alkoxide
Usage of alkoxide
anions such as sodium methoxide, instead of sodium hydroxide, yields the ring-contracted ester
product.
, 1918) not one but two halogen atoms flank the ketone resulting in a new contracted ketone after oxidation and decarboxylation
s (for instance those of ATP
) protected
by so-called p-hydroxyphenacyl groups. The deprotection proceeds through a triplet diradical
(3) and a dione
spiro
intermediate (4) although the latter has thus far eluded detection.
Favorskii reaction
The Favorskii reaction , named for the Russian chemist Alexei Yevgrafovich Favorskii, is a special case of nucleophilic attack on a carbonyl group involving a terminal alkyne with acidic protons....
), named for the Russian chemist Alexei Yevgrafovich Favorskii
Alexei Yevgrafovich Favorskii
Alexey Yevgrafovich Favorsky, also spelled Favorskii, was a Soviet/Russian chemist.-Life:Favorsky studied chemistry at the imperial University of Saint Petersburg from 1878 to 1882. He joined Alexander Butlerov's laboratory for several years, and in 1891 became a lecturer. In 1895, Favorksy...
, is most principally a rearrangement of cyclopropanone
Cyclopropanone
Cyclopropanone is an organic compound with molecular formula C3H4O consisting of a cyclopropane carbon framework with a ketone functional group. The parent compound is labile with melting point −90 °C and has been prepared by reaction of ketene with diazomethane at −145 °C...
s and α-halo ketones which leads to carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
derivatives. In the case of cyclic α-halo ketones, the Favorski rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
, to yield a carboxylic acid but most of the time either an alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
base or an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
to yield an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
or an amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
, respectively. α,α’-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.
In the case of cylic α-halo ketones, the rearrangement occurs as depicted below :

Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is thought to involve the formation of an enolate on the side of the ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
away from the chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
atom. This enolate cyclizes to a cyclopropanone
Cyclopropanone
Cyclopropanone is an organic compound with molecular formula C3H4O consisting of a cyclopropane carbon framework with a ketone functional group. The parent compound is labile with melting point −90 °C and has been prepared by reaction of ketene with diazomethane at −145 °C...
intermediate which is then attacked by the hydroxide nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
.

Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
anions such as sodium methoxide, instead of sodium hydroxide, yields the ring-contracted ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
product.
Wallach degradation
In the related Wallach degradation (Otto WallachOtto Wallach
Otto Wallach was a German chemist and recipient of the 1910 Nobel prize in Chemistry for his work on alicyclic compounds.-Biography:...
, 1918) not one but two halogen atoms flank the ketone resulting in a new contracted ketone after oxidation and decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
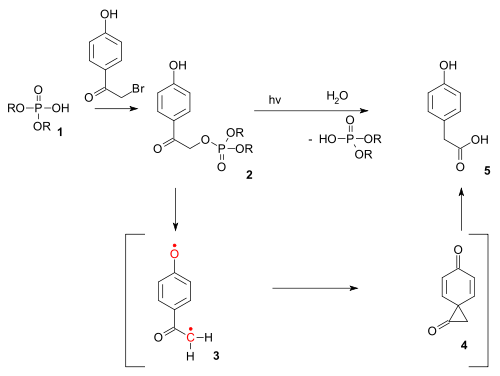
Photo-Favorskii reaction
The reaction type also exists as a photochemical reaction. The photo-Favorskii reaction has been used in the photochemical unlocking of certain phosphatePhosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
s (for instance those of ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
) protected
Protecting group
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction...
by so-called p-hydroxyphenacyl groups. The deprotection proceeds through a triplet diradical
Diradical
A diradical in organic chemistry is a molecular species with two electrons occupying two degenerate molecular orbitals . They are known by their higher reactivities and shorter lifetimes. In a broader definition diradicals are even-electron molecules that have one bond less than the number...
(3) and a dione
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
spiro
Spiro compound
A spiro compound is a bicyclic organic compound with rings connected through just one atom. The rings can be different in nature or identical. The connecting atom is also called the spiroatom, most often a quaternary carbon...
intermediate (4) although the latter has thus far eluded detection.