
Duhem–Margules equation
Encyclopedia
The Duhem–Margules equation, named for Pierre Duhem
and Max Margules
, is a thermodynamic statement of the relationship between the two components
of a single liquid
where the vapour mixture is regarded as an ideal gas
:
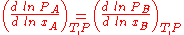
where PA and PB are the partial vapour pressures of the two constituents and xA and xB are the mole fractions of the liquid.
Pierre Duhem
Pierre Maurice Marie Duhem was a French physicist, mathematician and philosopher of science, best known for his writings on the indeterminacy of experimental criteria and on scientific development in the Middle Ages...
and Max Margules
Max Margules
-Life and career:Max Margules studied mathematics, physics, and chemistry in Vienna. In 1877 he joined, as volunteer, ZAMG in Vienna . After two years he left Vienna to study 1 year at Berlin. He returned to Vienna and received his Phd degree in the area of Electrodynamics. During his doctoral...
, is a thermodynamic statement of the relationship between the two components
Component (thermodynamics)
In thermodynamics, a component is a chemically distinct constituent ofa system. Calculating the number of components in a system is necessary, for example, when applying Gibbs phase rule in determination of the number of degrees of freedom of a system....
of a single liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
where the vapour mixture is regarded as an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
:
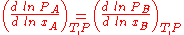
where PA and PB are the partial vapour pressures of the two constituents and xA and xB are the mole fractions of the liquid.
Sources
- Atkins, PeterPeter AtkinsPeter William Atkins is a British chemist and former Professor of Chemistry at the University of Oxford and a Fellow of Lincoln College. He is a prolific writer of popular chemistry textbooks, including Physical Chemistry, Inorganic Chemistry, and Molecular Quantum Mechanics...
and Julio de Paula. 2002. Physical Chemistry, 7th ed. New York: W. H. Freeman and Co. - Carter, Ashley H. 2001. Classical and Statistical Thermodynamics. Upper Saddle River: Prentice Hall.
- Harris, Joseph. "The Duhem–Margules Equation." Algebraic Geometry. Harvard Science Center, Cambridge. 19 Oct. 2009. Lecture.

