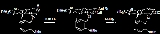
Diphenyl diselenide
Encyclopedia
Diphenyl diselenide is the chemical compound
with the formula (C6H5)2Se2, abbreviated Ph2Se2 This orange-coloured solid is the oxidized derivative of benzeneselenol
. It is used as a source of the PhSe unit in organic synthesis
.
Ph2Se2 is prepared by the oxidation of benzeneselenoate, which is generated via the Grignard reagent:
It has a centrosymmetric structure, with an Se-Se bond length of 2.29 A.
PhSeNa is a useful nucleophile, and can be used to introduce the phenylselenyl group by nucleophilic substitution
of alkyl halides, alkyl sulfonates (mesylates or tosyl
ates) or epoxide
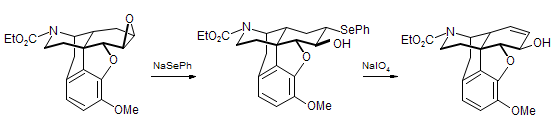
s. The example below was taken from a synthesis of morphine.
PhSeCl is a powerful electrophile, used to introduce PhSe groups by reaction with a variety of nucleophiles, including enolates, enol silyl ethers, Grignard reagents, organolithium reagent
s, alkene
s and amine
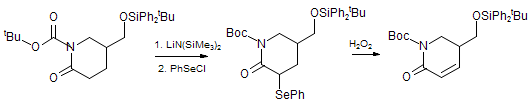
s. In the sequence below (early steps in the synthesis of Strychnofoline), a PhSe group is introduced by reaction of a lactam
enolate with PhSeCl. This sequence is a powerful method for the conversion of carbonyl compounds to their α,β-unsaturated analogs.
Diphenyl diselenide itself is also a source of a weakly electrophilic PhSe group in reactions with relatively powerful nucleophiles like Grignard reagents, lithium reagents and ester enolates (but not ketone enolates or weaker nucleophiles). PhSeCl is both more reactive, and more efficient, since with Ph2Se2 half of the selenium is wasted.
N-Phenylselenophtalimide (N-PSP) can be used if PhSeCl is too strong and diphenyl diselenide is too weak or wasteful.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula (C6H5)2Se2, abbreviated Ph2Se2 This orange-coloured solid is the oxidized derivative of benzeneselenol
Benzeneselenol
Benzeneselenol is the chemical compound with the formula C6H5SeH, often abbreviated PhSeH. This intensely malodorous liquid is a useful reagent in organic synthesis.-Synthesis and basic properties:...
. It is used as a source of the PhSe unit in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
Ph2Se2 is prepared by the oxidation of benzeneselenoate, which is generated via the Grignard reagent:
- PhMgBrPhenylmagnesium bromidePhenylmagnesium bromide, with the simplified formula , is a magnesium-containing organometallic compound. It is so commonly used that it is commercially available as a solution in diethyl ether or tetrahydrofuran . Phenylmagnesium bromide is a Grignard reagent...
+ Se → PhSeMgBr - 2 PhSeMgBr + Br2 → Ph2Se2 + 2 MgBr2Magnesium bromideMagnesium bromide is a chemical compound of magnesium and bromine that is white and deliquescent. It is often used as a mild sedative and as an anticonvulsant for treatment of nervous disorders. It is water soluble and somewhat soluble in alcohol...
It has a centrosymmetric structure, with an Se-Se bond length of 2.29 A.
Reactions
Two reactions characteristic of Ph2Se2 are reduction and chlorination:- Ph2Se2 + 2 Na → 2 PhSeNa
- Ph2Se2 + Cl2 → 2 PhSeCl
PhSeNa is a useful nucleophile, and can be used to introduce the phenylselenyl group by nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
of alkyl halides, alkyl sulfonates (mesylates or tosyl
Tosyl
A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid...
ates) or epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
s. The example below was taken from a synthesis of morphine.
PhSeCl is a powerful electrophile, used to introduce PhSe groups by reaction with a variety of nucleophiles, including enolates, enol silyl ethers, Grignard reagents, organolithium reagent
Organolithium reagent
An organolithium reagent is an organometallic compound with a direct bond between a carbon and a lithium atom. As the electropositive nature of lithium puts most of the charge density of the bond on the carbon atom, effectively creating a carbanion, organolithium compounds are extremely powerful...
s, alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s and amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s. In the sequence below (early steps in the synthesis of Strychnofoline), a PhSe group is introduced by reaction of a lactam
Lactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
enolate with PhSeCl. This sequence is a powerful method for the conversion of carbonyl compounds to their α,β-unsaturated analogs.
Diphenyl diselenide itself is also a source of a weakly electrophilic PhSe group in reactions with relatively powerful nucleophiles like Grignard reagents, lithium reagents and ester enolates (but not ketone enolates or weaker nucleophiles). PhSeCl is both more reactive, and more efficient, since with Ph2Se2 half of the selenium is wasted.
- Ph2Se2 + Nu− → PhSeNu + PhSe−
N-Phenylselenophtalimide (N-PSP) can be used if PhSeCl is too strong and diphenyl diselenide is too weak or wasteful.