
Dimethylphenylphosphine
Encyclopedia
Dimethylphenylphosphine is a organophosphorus compound with a formula P(C6H5)(CH3)2. The phosphorus
is connected to a phenyl group and two methyl groups. This colorless air sensitive liquid is commonly used as a ligand
in transition metal complexes. These complexes are often soluble in organic solvents.
with dichlorophenylphosphine
.
Cl2P + 2CH3MgBr → (C6H5)(CH3)2P + 2MgBrCl
The phosphine
is purified by distillation under reduced pressure.
A solution of (C6H5)(CH3)2P in CDCl3 shows proton NMR
signals at δ = 7.0-7.5 and a doublet at δ = 1.2. The phosphorus-31 NMR
spectrum shows a singlet at -45.89 ppm in CDCl3.
and angles are the following: P-CMe: 1.844, P-CPh: 1.845 Å, C-C: 1.401 Å, C-HMe: 1.090 Å, C-HPh: 1.067 Å, C-P-C: 96.9°, C-P-C (ring): 103.4°, P-C-H: 115.2°.
Also when comparing the acidity of HPR3 (PR3 = PMe2Ph, PPh3, PEt3) in CD2Cl2, the resulting pKa
value is the following: [HPMe2Ph]+= 6.8, [HPPh3]+ = 2.7, [HPEt3]+ = 8.7. Thus PMe2Ph is intermediate in basicity relative to PPh3 and PEt3.
The ligand cone angle (θ) is the apex angle of a cylindrical cone, which is centered 2.28 Å from the center of the P atom. However, the cone angle of an unsymmetrical ligand cannot be determined in the same. In order to determine an effective cone angle for an unsymmetrical ligand PX1X2X3, the following equation is used:
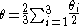
Where θi represent the half angle.
The resulting angles for -PMe3, PMe2Ph, PPh3 are the following:
PMe3= 118°, PMe2Ph= 122°, PPh3= 145°. Thus PMe2Ph is intermediate in size relative to PMe3 and PPh3.
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
is connected to a phenyl group and two methyl groups. This colorless air sensitive liquid is commonly used as a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
in transition metal complexes. These complexes are often soluble in organic solvents.
Preparation
Dimethylphenylphosphine is prepared by the reaction of methylmagnesium halideGrignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
with dichlorophenylphosphine
Dichlorophenylphosphine
Dichlorophenylphosphine is an organophosphorus compound with the formula C6H5PCl2. This colourless viscous liquid is commonly used in the synthesis of phosphine ligands....
.
Cl2P + 2CH3MgBr → (C6H5)(CH3)2P + 2MgBrCl
The phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
is purified by distillation under reduced pressure.
A solution of (C6H5)(CH3)2P in CDCl3 shows proton NMR
Proton NMR
Proton NMR is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen is used, practically all of the hydrogen consists of the...
signals at δ = 7.0-7.5 and a doublet at δ = 1.2. The phosphorus-31 NMR
Phosphorus-31 NMR
Phosphorus-31 NMR spectroscopy is an analytical technique. Solution 31P-NMR is one of the more routine NMR techniques because 31P has an isotopic abundance of 100% and a relatively high magnetogyric ratio. The 31P nucleus also has a spin of ½, making spectra relatively easy to interpret...
spectrum shows a singlet at -45.89 ppm in CDCl3.
Structure and properties
Dimethylphenylphosphine is a pyramidal molecule where the phenyl group and two methyl groups are connected to the phosphorus. The bond lengthBond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
and angles are the following: P-CMe: 1.844, P-CPh: 1.845 Å, C-C: 1.401 Å, C-HMe: 1.090 Å, C-HPh: 1.067 Å, C-P-C: 96.9°, C-P-C (ring): 103.4°, P-C-H: 115.2°.
Comparisons with related phosphine ligands
The υCO of Ir-Cl(CO)(PPh3)2 and Ir-Cl(CO)(PMe2Ph)2 are both at 1960 cm-1, whereas the υCO for Ir-Cl(CO)(PMe3)2 is at 1938 cm-1.Also when comparing the acidity of HPR3 (PR3 = PMe2Ph, PPh3, PEt3) in CD2Cl2, the resulting pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
value is the following: [HPMe2Ph]+= 6.8, [HPPh3]+ = 2.7, [HPEt3]+ = 8.7. Thus PMe2Ph is intermediate in basicity relative to PPh3 and PEt3.
The ligand cone angle (θ) is the apex angle of a cylindrical cone, which is centered 2.28 Å from the center of the P atom. However, the cone angle of an unsymmetrical ligand cannot be determined in the same. In order to determine an effective cone angle for an unsymmetrical ligand PX1X2X3, the following equation is used:
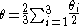
Where θi represent the half angle.
The resulting angles for -PMe3, PMe2Ph, PPh3 are the following:
PMe3= 118°, PMe2Ph= 122°, PPh3= 145°. Thus PMe2Ph is intermediate in size relative to PMe3 and PPh3.

