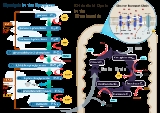
Cellular respiration
Overview
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
reactions and processes that take place in the cell
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
s of organism
Organism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
s to convert biochemical energy from nutrients into adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP), and then release waste products. The reactions involved in respiration are catabolic reactions
Catabolism
Catabolism is the set of metabolic pathways that break down molecules into smaller units and release energy. In catabolism, large molecules such as polysaccharides, lipids, nucleic acids and proteins are broken down into smaller units such as monosaccharides, fatty acids, nucleotides, and amino...
that involve the redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reaction (oxidation
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of one molecule and the reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of another). Respiration is one of the key ways a cell gains useful energy to fuel cellular changes
Nutrients that are commonly used by animal and plant cells in respiration include sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
, amino acids and fatty acids, and a common oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
(electron acceptor
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
) is molecular oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(O2).
Unanswered Questions
Discussions

