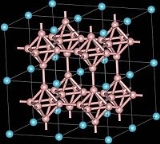
Yttrium borides
Encyclopedia
Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity
at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode
. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating
for low-energy synchrotron
radiation (1–2 keV).
 Yttrium diboride has the same hexagonal crystal structure as aluminium diboride
Yttrium diboride has the same hexagonal crystal structure as aluminium diboride
and magnesium diboride
– an important superconducting material. Its Pearson symbol
is hP3, space group
P6/mmm (No 191), a = 0.3289 nm, b = 0.3289 nm, c = 0.3843 nm and the calculated density is 5.1 g/cm3. In this structure, the boron atoms form graphite like sheets with yttrium atoms between them. YB2 crystals are unstable to moderate heating in air – they start oxidizing at 400 °C and completely oxidize at 800 °C. YB2 melts at ~2220 °C.
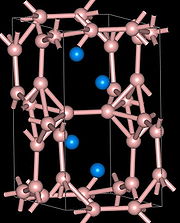 YB4 has tetragonal crystal structure with space group
YB4 has tetragonal crystal structure with space group
P4/mbm (No. 127), Pearson symbol tP20, a = 0.711 nm, b = 0.711 nm, c = 0.4019 nm, calculated density 4.32 g/cm3. High-quality YB4 crystals of few centimeters in size can be grown by the multiple-pass floating zone technique.
, LaB6
, etc., see infobox). High-quality YB6 crystals of few centimeters in size can be grown by the multiple-pass floating zone technique. YB6 is a superconductor with the relatively high transition temperature (onset) of 8.4 K.
cF52, space group
Fmm (No. 225), a = 0.7468 nm. Its structural unit is 12 cuboctahedron
. The Debye temperature of YB12 is ~1040 K, and it is not superconducting at temperatures above 2.5 K.
. The boron framework of YB25 is one of the simplest among icosahedron-based borides – it consists of only one kind of icosahedra and one bridging boron site. The bridging boron site is tetrahedrally coordinated by four boron atoms. Those atoms are another boron atom in the counter bridge site and three equatorial boron atoms of one of three B12 icosahedra. The yttrium sites have partial occupancies of about 60–70%, and the YB25 formula merely reflects the average atomic ratio [B]/[Y] = 25. Both the Y atoms and B12 icosahedra form zigzags along the x-axis. The bridging boron atoms connect three equatorial boron atoms of three icosahedra and those icosahedra make up a network parallel to the (101) crystal plane (x-z plane in the figure). The bonding distance between the bridging boron and the equatorial boron atoms is 0.1755 nm, which is typical for the strong covalent B-B bond (bond length 0.17–0.18 nm); thus, the bridging boron atoms strengthen the individual network planes. On the other hand, the large distance between the boron atoms within the bridge (0.2041 nm) reveals a weaker interaction, and thus the bridging sites contribute little to the bonding between the network planes.
YB25 crystals can be grown by heating a compressed pellet of yttria (Y2O3) and boron powder to ~1700 0C. The YB25 phase is stable up to 1850 °C. Above this temperature it decomposes into YB12 and YB66 without melting. This makes it difficult to grow a single crystal of YB25 by the melt growth method.
 YB66 was discovered in 1960 and its structure was solved in 1969. The structure is face-centered cubic
YB66 was discovered in 1960 and its structure was solved in 1969. The structure is face-centered cubic
, with space group Fmc (No. 226), Pearson symbol cF1936 and lattice constant a = 2.3440(6) nm. There are 13 boron sites B1–B13 and one yttrium site. The B1 sites form one B12 icosahedron
and the B2–B9 sites make up another icosahedron. These icosahedra arrange in a thirteen-icosahedron unit (B12)12B12 which is called supericosahedron. The icosahedron formed by the B1 site atoms is located at the center of the supericosahedron. The supericosahedron is one of the basic units of the boron framework of YB66. There are two types of supericosahedra: one occupies the cubic face centers and another, which is rotated by 90°, is located at the center of the cell and at the cell edges. Thus, there are eight supericosahedra (1248 boron atoms) in the unit cell.
Another structure unit of YB66 is B80 cluster of 80 boron sites formed by the B10 to B13 sites. All those 80 sites are partially occupied and in total contain only about 42 boron atoms. The B80 cluster is located at the body center of the octant of the unit cell, i.e., at the 8a position (1/4, 1/4, 1/4); thus, there are eight such clusters (336 boron atoms) per unit cell. Two independent structure analyses came to the same conclusion that the total number of boron atoms in the unit cell is 1584. The boron framework structure of YB66 is shown in the figure to the right. A schematic drawing under it indicates relative orientations of the supericosahedra, and the B80 clusters are depicted by light green and dark green spheres, respectively; at the top surface of the unit cell, the relative orientations of the supericosahedra are indicated by arrows. There are 48 yttrium sites ((0.0563, 1/4, 1/4) for YB62) in the unit cell. Fixing the occupancy of the Y site to 0.5 results in 24 Y atoms in the unit cell and the chemical composition of YB66; this occupancy of 0.5 implies that the yttrium pair has always one Y atom with one empty site.
YB66 has density 2.52 g/cm3, low thermal conductivity of 0.02 W/(cm·K), elastic constants c11 = 3.8×109 and c44 = 1.6×109 Newton/m2 and Debye temperature of 1300 K. As all yttrium borides, YB66 is a hard material and exhibits Knoop hardness of 26 GPa.
High-quality YB66 crystals of few centimeters in size can be grown by the multiple-pass floating zone technique and be used as X-ray monochromators.
The large unit cell of YB66 results in large lattice constant of 2.344 nm. This property, together with high thermal and mechanical stability resulted in application of YB66 as dispersive elements of X-ray monochromators for low energy radiation (1–2 keV).
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode
Hot cathode
In vacuum tubes, a hot cathode is a cathode electrode which emits electrons due to thermionic emission. In the accelerator community, these are referred to as thermionic cathodes. The heating element is usually an electrical filament...
. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating
Diffraction grating
In optics, a diffraction grating is an optical component with a periodic structure, which splits and diffracts light into several beams travelling in different directions. The directions of these beams depend on the spacing of the grating and the wavelength of the light so that the grating acts as...
for low-energy synchrotron
Synchrotron
A synchrotron is a particular type of cyclic particle accelerator in which the magnetic field and the electric field are carefully synchronised with the travelling particle beam. The proton synchrotron was originally conceived by Sir Marcus Oliphant...
radiation (1–2 keV).
YB2 (yttrium diboride)

Aluminium diboride
Aluminium diboride is a chemical compound made from the metal aluminium and the non-metal boron. It is one of two compounds of aluminium and boron, the other being AlB12 that are both commonly referred to as aluminium boride....
and magnesium diboride
Magnesium diboride
Magnesium diboride is a simple ionic binary compound that has proven to be an inexpensive and useful superconducting material.Its superconductivity was announced in the journal Nature in March 2001. Its critical temperature of is the highest amongst conventional superconductors...
– an important superconducting material. Its Pearson symbol
Pearson symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W.B. Pearson. The symbol is made up of two letters followed by a number. For example:* Diamond structure, cF8...
is hP3, space group
Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
P6/mmm (No 191), a = 0.3289 nm, b = 0.3289 nm, c = 0.3843 nm and the calculated density is 5.1 g/cm3. In this structure, the boron atoms form graphite like sheets with yttrium atoms between them. YB2 crystals are unstable to moderate heating in air – they start oxidizing at 400 °C and completely oxidize at 800 °C. YB2 melts at ~2220 °C.
YB4 (yttrium tetraboride)

Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
P4/mbm (No. 127), Pearson symbol tP20, a = 0.711 nm, b = 0.711 nm, c = 0.4019 nm, calculated density 4.32 g/cm3. High-quality YB4 crystals of few centimeters in size can be grown by the multiple-pass floating zone technique.
YB6 (yttrium hexaboride)
YB6 is a black odorless powder having density of 3.67 g/cm3; it has the same cubic crystalline structure as other hexaborides (CaB6Calcium hexaboride
Calcium hexaboride is a compound of calcium and boron with the chemical formula CaB6. It is an important material due to its high electrical conductivity, hardness, chemical stability, and melting point. It is a black, lustrous, chemically inert powder with a low density...
, LaB6
Lanthanum hexaboride
]]Lanthanum hexaboride is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material that has a melting point of 2210 °C, and is insoluble in water and hydrochloric acid. It has a low work function and one of the highest electron emissivities known, and is stable in...
, etc., see infobox). High-quality YB6 crystals of few centimeters in size can be grown by the multiple-pass floating zone technique. YB6 is a superconductor with the relatively high transition temperature (onset) of 8.4 K.
YB12 (yttrium dodecaboride)
YB12 crystals have a cubic structure with density of 3.44 g/cm3, Pearson symbolPearson symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W.B. Pearson. The symbol is made up of two letters followed by a number. For example:* Diamond structure, cF8...
cF52, space group
Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
Fmm (No. 225), a = 0.7468 nm. Its structural unit is 12 cuboctahedron
Cuboctahedron
In geometry, a cuboctahedron is a polyhedron with eight triangular faces and six square faces. A cuboctahedron has 12 identical vertices, with two triangles and two squares meeting at each, and 24 identical edges, each separating a triangle from a square. As such it is a quasiregular polyhedron,...
. The Debye temperature of YB12 is ~1040 K, and it is not superconducting at temperatures above 2.5 K.
YB25
The structure of yttrium borides with B/Y ratio of 25 and above consists of a network of B12 icosahedraIcosahedron
In geometry, an icosahedron is a regular polyhedron with 20 identical equilateral triangular faces, 30 edges and 12 vertices. It is one of the five Platonic solids....
. The boron framework of YB25 is one of the simplest among icosahedron-based borides – it consists of only one kind of icosahedra and one bridging boron site. The bridging boron site is tetrahedrally coordinated by four boron atoms. Those atoms are another boron atom in the counter bridge site and three equatorial boron atoms of one of three B12 icosahedra. The yttrium sites have partial occupancies of about 60–70%, and the YB25 formula merely reflects the average atomic ratio [B]/[Y] = 25. Both the Y atoms and B12 icosahedra form zigzags along the x-axis. The bridging boron atoms connect three equatorial boron atoms of three icosahedra and those icosahedra make up a network parallel to the (101) crystal plane (x-z plane in the figure). The bonding distance between the bridging boron and the equatorial boron atoms is 0.1755 nm, which is typical for the strong covalent B-B bond (bond length 0.17–0.18 nm); thus, the bridging boron atoms strengthen the individual network planes. On the other hand, the large distance between the boron atoms within the bridge (0.2041 nm) reveals a weaker interaction, and thus the bridging sites contribute little to the bonding between the network planes.
YB25 crystals can be grown by heating a compressed pellet of yttria (Y2O3) and boron powder to ~1700 0C. The YB25 phase is stable up to 1850 °C. Above this temperature it decomposes into YB12 and YB66 without melting. This makes it difficult to grow a single crystal of YB25 by the melt growth method.
YB50
YB50 crystals have orthorhombic structure with space group P21212 (No. 18), a = 1.66251 nm, b = 1.76198 nm, c = 0.94797 nm. They can be grown by heating a compressed pellet of yttria (Y2O3) and boron powder to ~1700 0C. Above this temperature YB50 decomposes into YB12 and YB66 without melting. This makes it difficult to grow a single crystal of YB50 by the melt growth method. Rare earth elements from Tb to Lu can also crystallize in the M50 form.YB66

Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
, with space group Fmc (No. 226), Pearson symbol cF1936 and lattice constant a = 2.3440(6) nm. There are 13 boron sites B1–B13 and one yttrium site. The B1 sites form one B12 icosahedron
Icosahedron
In geometry, an icosahedron is a regular polyhedron with 20 identical equilateral triangular faces, 30 edges and 12 vertices. It is one of the five Platonic solids....
and the B2–B9 sites make up another icosahedron. These icosahedra arrange in a thirteen-icosahedron unit (B12)12B12 which is called supericosahedron. The icosahedron formed by the B1 site atoms is located at the center of the supericosahedron. The supericosahedron is one of the basic units of the boron framework of YB66. There are two types of supericosahedra: one occupies the cubic face centers and another, which is rotated by 90°, is located at the center of the cell and at the cell edges. Thus, there are eight supericosahedra (1248 boron atoms) in the unit cell.
Another structure unit of YB66 is B80 cluster of 80 boron sites formed by the B10 to B13 sites. All those 80 sites are partially occupied and in total contain only about 42 boron atoms. The B80 cluster is located at the body center of the octant of the unit cell, i.e., at the 8a position (1/4, 1/4, 1/4); thus, there are eight such clusters (336 boron atoms) per unit cell. Two independent structure analyses came to the same conclusion that the total number of boron atoms in the unit cell is 1584. The boron framework structure of YB66 is shown in the figure to the right. A schematic drawing under it indicates relative orientations of the supericosahedra, and the B80 clusters are depicted by light green and dark green spheres, respectively; at the top surface of the unit cell, the relative orientations of the supericosahedra are indicated by arrows. There are 48 yttrium sites ((0.0563, 1/4, 1/4) for YB62) in the unit cell. Fixing the occupancy of the Y site to 0.5 results in 24 Y atoms in the unit cell and the chemical composition of YB66; this occupancy of 0.5 implies that the yttrium pair has always one Y atom with one empty site.
YB66 has density 2.52 g/cm3, low thermal conductivity of 0.02 W/(cm·K), elastic constants c11 = 3.8×109 and c44 = 1.6×109 Newton/m2 and Debye temperature of 1300 K. As all yttrium borides, YB66 is a hard material and exhibits Knoop hardness of 26 GPa.
High-quality YB66 crystals of few centimeters in size can be grown by the multiple-pass floating zone technique and be used as X-ray monochromators.
The large unit cell of YB66 results in large lattice constant of 2.344 nm. This property, together with high thermal and mechanical stability resulted in application of YB66 as dispersive elements of X-ray monochromators for low energy radiation (1–2 keV).

