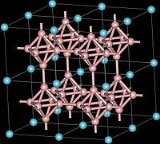
Calcium hexaboride
Encyclopedia
Calcium hexaboride is a compound of calcium
and boron
with the chemical formula CaB6. It is an important material due to its high electrical conductivity, hardness, chemical stability, and melting point
. It is a black, lustrous, chemically inert powder with a low density. It has the cubic structure typical for metal hexaborides, with octahedral units of 6 boron atoms combined with calcium atoms. CaB6 and lanthanum
doped CaB6 show weak ferromagnetic
properties, which is a remarkable fact because calcium and boron are neither magnetic, nor have inner 3d or 4f electronic shells, which are usually required for ferromagnetism.
, valence fluctuation and Kondo effect
s. However, the most remarkable property of CaB6 is its ferromagnetism. It occurs at unexpectedly high temperature (600 K) and with low magnetic moment (below 0.07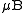 per atom). The origin of this high temperature ferromagnetism is the ferromagnetic phase of a dilute electron gas, linkage to the presumed excitonic state in calcium boride, or external impurities on the surface of the sample. The impurities might include Iron
per atom). The origin of this high temperature ferromagnetism is the ferromagnetic phase of a dilute electron gas, linkage to the presumed excitonic state in calcium boride, or external impurities on the surface of the sample. The impurities might include Iron
and Nickel
probably coming from boron in sample preparation.
CaB6 is insoluble in H2O, MeOH (methanol), and EtOH (ethanol) and dissolves slowly in acids. Its microhardness is 27 GPa, Knoop hardness is 2600 kg/mm2), Young modulus is 379 GPa, and electrical resistivity is greater than 2·1010 Ω·m for pure crystals. CaB6 is a semiconductor with an energy gap estimated as 1.0 eV. The low, semi-metallic conductivity of many CaB6 samples can be explained by unintentional doping due to impurities and possible non-stoichiometry.
43Ca NMR data contains δpeak at -56.0 ppm and δiso at -41.3 ppm where δiso is taken as peak max +0.85 width, the negative shift is due to the high coordination number.
Raman Data: Calcium hexaboride has three Raman peaks at 754.3, 1121.8, and 1246.9 cm−1 due to the active modes A1g, Eg, and T2g respectively.
Observed Vibrational Frequencies cm−1 : 1270(strong) from A1g stretch, 1154 (med.) and 1125(shoulder) from Eg stretch, 526, 520, 485, and 470 from F1g rotation, 775 (strong) and 762 (shoulder) from F2g bend, 1125 (strong) and 1095 (weak)from F1u bend, 330 and 250 from F1u translation, and 880 (med.) and 779 from F2u bend.
Other methods of producing CaB6 powder include:
results in relatively poor quality material.
ed steel
and as a deoxidation agent in production of oxygen-free copper
. The latter results in higher conductivity than conventionally phosphorus-deoxidized copper owing to the low solubility of boron in copper. CaB6 can also serve as a high temperature material, surface protection, abrasive
s, tools, and wear resistant material.
CaB6 is highly conductive, has low work function, and thus can be used as a cathode material. When used at elevated temperature, calcium hexaboride will oxidize degrading its properties and shortening its usable lifespan.
CaB6 is also a promising candidate for n-type thermoelectric materials, because its power factor is larger than or comparable to that of common thermoelectric materials Bi2Te3 and PbTe.
CaB also can be used as an antioxidant in carbon bonded refractories.
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
and boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
with the chemical formula CaB6. It is an important material due to its high electrical conductivity, hardness, chemical stability, and melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
. It is a black, lustrous, chemically inert powder with a low density. It has the cubic structure typical for metal hexaborides, with octahedral units of 6 boron atoms combined with calcium atoms. CaB6 and lanthanum
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57.Lanthanum is a silvery white metallic element that belongs to group 3 of the periodic table and is the first element of the lanthanide series. It is found in some rare-earth minerals, usually in combination with cerium and...
doped CaB6 show weak ferromagnetic
Ferromagnetism
Ferromagnetism is the basic mechanism by which certain materials form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished...
properties, which is a remarkable fact because calcium and boron are neither magnetic, nor have inner 3d or 4f electronic shells, which are usually required for ferromagnetism.
Properties
CaB6 has been investigated in the past due to a variety of peculiar physical properties, such as superconductivitySuperconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
, valence fluctuation and Kondo effect
Kondo effect
In physics, the Kondo effect describes the scattering of conduction electrons in a metal due to magnetic impurities. It is a measure of how electrical resistivity changes with temperature....
s. However, the most remarkable property of CaB6 is its ferromagnetism. It occurs at unexpectedly high temperature (600 K) and with low magnetic moment (below 0.07
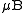 per atom). The origin of this high temperature ferromagnetism is the ferromagnetic phase of a dilute electron gas, linkage to the presumed excitonic state in calcium boride, or external impurities on the surface of the sample. The impurities might include Iron
per atom). The origin of this high temperature ferromagnetism is the ferromagnetic phase of a dilute electron gas, linkage to the presumed excitonic state in calcium boride, or external impurities on the surface of the sample. The impurities might include IronIron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
and Nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
probably coming from boron in sample preparation.
CaB6 is insoluble in H2O, MeOH (methanol), and EtOH (ethanol) and dissolves slowly in acids. Its microhardness is 27 GPa, Knoop hardness is 2600 kg/mm2), Young modulus is 379 GPa, and electrical resistivity is greater than 2·1010 Ω·m for pure crystals. CaB6 is a semiconductor with an energy gap estimated as 1.0 eV. The low, semi-metallic conductivity of many CaB6 samples can be explained by unintentional doping due to impurities and possible non-stoichiometry.
Structural Information
The crystal structure of calcium hexaboride is a cubic lattice with calcium at the cell centre and compact, regular octahedra of boron atoms linked at the vertices by B-B bonds to give a three-dimensional boron network. Each calcium has 24 nearest-neighbor boron atoms The calcium atoms are arranged in simple cubic packing so that there are holes between groups of eight calcium atoms situated at the vertices of a cube. The simple cubic structure is expanded by the introduction of the octahedral B6 groups and the structure is a CsCl-like packing of the calcium and hexaboride groups. Another way of describing calcium hexaboride is as having a metal and a B62- octahedral polymeric anions in a CsCl-type structure were the Calcium atoms occupy the Cs sites and the B6 octahedra in the Cl sites. The Ca-B bond length is 3.05 Å and the B-B bond length is 1.7 Å.43Ca NMR data contains δpeak at -56.0 ppm and δiso at -41.3 ppm where δiso is taken as peak max +0.85 width, the negative shift is due to the high coordination number.
Raman Data: Calcium hexaboride has three Raman peaks at 754.3, 1121.8, and 1246.9 cm−1 due to the active modes A1g, Eg, and T2g respectively.
Observed Vibrational Frequencies cm−1 : 1270(strong) from A1g stretch, 1154 (med.) and 1125(shoulder) from Eg stretch, 526, 520, 485, and 470 from F1g rotation, 775 (strong) and 762 (shoulder) from F2g bend, 1125 (strong) and 1095 (weak)from F1u bend, 330 and 250 from F1u translation, and 880 (med.) and 779 from F2u bend.
Preparation
- One of the main reactions for industrial production is:
- CaO + 3 B2O3 + 10 Mg → CaB6 + 10 MgO
Other methods of producing CaB6 powder include:
- Direct reaction of calcium or calcium oxideCalcium oxideCalcium oxide , commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline crystalline solid at room temperature....
and boron at 1000°C;
- Ca + 6B → CaB6
- Reacting Ca(OH)2 with boron in vacuum at about 1700 °C (carbothermal reduction);
- Ca(OH)2 +7B → CaB6 + BO(g) + H2O(g)
- Reacting calcium carbonateCalcium carbonateCalcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks in all parts of the world, and is the main component of shells of marine organisms, snails, coal balls, pearls, and eggshells. Calcium carbonate is the active ingredient in agricultural lime,...
with boron carbideBoron carbideBoron carbide is an extremely hard boron–carbon ceramic material used in tank armor, bulletproof vests, and numerous industrial applications...
in vacuum at above 1400 °C (carbothermal reduction)
- Reacting of CaO and H3BO3 and Mg to 1100°C.
- Low-temperature (500 °C) synthesis
- CaCl2 + 6NaBH4 → CaB6 + 2NaCl + 12H2 + 4Na
results in relatively poor quality material.
- To produce pure CaB6 single crystals, e.g., for use as cathode material, the thus obtained CaB6 powder is further recrystallized and purified with the zone meltingZone meltingZone melting is a group of similar methods of purifying crystals, in which a narrow region of a crystal is molten, and this molten zone is moved along the crystal...
technique. The typical growth rate is 30 cm/h and crystal size ~1x10 cm.
- Single-crystal CaB6 Nanowires (diameter 15-40 nm, length 1-10 micrometres) can be obtained by pyrolysis of diboraneDiboraneDiborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
(B2H6) over calcium oxide (CaO) powders at 860-900 °C, in presence of Ni catalyst.
Uses
Calcium hexaboride is used in the manufacturing of boron-alloyAlloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
ed steel
Steel
Steel is an alloy that consists mostly of iron and has a carbon content between 0.2% and 2.1% by weight, depending on the grade. Carbon is the most common alloying material for iron, but various other alloying elements are used, such as manganese, chromium, vanadium, and tungsten...
and as a deoxidation agent in production of oxygen-free copper
Oxygen-free copper
Oxygen-free copper or Oxygen-free high thermal conductivity copper generally refers to a group of wrought high conductivity copper alloys that have been electrolytically refined to reduce the level of oxygen to .001% or below....
. The latter results in higher conductivity than conventionally phosphorus-deoxidized copper owing to the low solubility of boron in copper. CaB6 can also serve as a high temperature material, surface protection, abrasive
Abrasive
An abrasive is a material, often a mineral, that is used to shape or finish a workpiece through rubbing which leads to part of the workpiece being worn away...
s, tools, and wear resistant material.
CaB6 is highly conductive, has low work function, and thus can be used as a cathode material. When used at elevated temperature, calcium hexaboride will oxidize degrading its properties and shortening its usable lifespan.
CaB6 is also a promising candidate for n-type thermoelectric materials, because its power factor is larger than or comparable to that of common thermoelectric materials Bi2Te3 and PbTe.
CaB also can be used as an antioxidant in carbon bonded refractories.

