
Thioketone
Encyclopedia
Thioketones are organosulfur compounds related to conventional ketone
s. Instead of the formula R2C=O, thioketones have the formula R2C=S. Unhindered alkylthioketones are typically unstable; such compounds tend to form polymers or rings
.
. Lawesson's reagent
, a derivative of P4S10, can also be used. Other methods uses a mixture of hydrogen chloride
with hydrogen sulfide
. Bis(trimethylsilyl)sulfide
has also been employed.
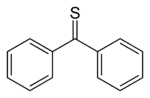 Thiobenzophenone
Thiobenzophenone
, (C6H5)2CS, is a stable deep blue
compound that dissolves readily in organic solvents. It photooxidizes in air to benzophenone
and sulfur. Since its discovery, a variety of related thiones have been prepared.
versions of the thioketones are less stable than thiones. Selenobenzophenone reversibly dimerizes. It is known to undergo cycloaddition with 1,3-dienes in a reaction similar to the Diels-Alder reaction.
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s. Instead of the formula R2C=O, thioketones have the formula R2C=S. Unhindered alkylthioketones are typically unstable; such compounds tend to form polymers or rings
Cyclic compound
In chemistry, a cyclic compound is a compound in which a series of atoms is connected to form a loop or ring.While the vast majority of cyclic compounds are organic, a few inorganic substances form cyclic compounds as well, including sulfur, silanes, phosphanes, phosphoric acid, and triboric acid. ...
.
Preparative methods
Thiones are usually prepared from ketones. A common reagent is phosphorus pentasulfidePhosphorus pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula P4S10. This yellow solid is the one of two phosphorus sulfides of commercial value...
. Lawesson's reagent
Lawesson's reagent
Lawesson's reagent, or LR, is a chemical compound used in organic synthesis as a thiation agent. Lawesson's reagent was first made popular by Sven-Olov Lawesson, who did not, however, invent it. Lawesson's reagent was first made in 1956 during a systematic study of the reactions of arenes with...
, a derivative of P4S10, can also be used. Other methods uses a mixture of hydrogen chloride
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
with hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
. Bis(trimethylsilyl)sulfide
Bis(trimethylsilyl)sulfide
Bis sulfide is the chemical compound with the formula 2S. Often abbreviated 2S, this colourless, vile-smelling liquid is a useful aprotic source of “S2−“ in chemical synthesis.-Synthesis:...
has also been employed.
Thiobenzophenone, the prototype

Thiobenzophenone
Thiobenzophenone is an organosulfur compound with the formula 2CS. It is the prototypical thioketone. Unlike other thioketones that tend to dimerize to form rings and polymers, thiobenzophenone is quite stable, although it photoxidizes in air to form benzophenone and sulfur...
, (C6H5)2CS, is a stable deep blue
Blue
Blue is a colour, the perception of which is evoked by light having a spectrum dominated by energy with a wavelength of roughly 440–490 nm. It is considered one of the additive primary colours. On the HSV Colour Wheel, the complement of blue is yellow; that is, a colour corresponding to an equal...
compound that dissolves readily in organic solvents. It photooxidizes in air to benzophenone
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
and sulfur. Since its discovery, a variety of related thiones have been prepared.
Selones
The seleniumSelenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
versions of the thioketones are less stable than thiones. Selenobenzophenone reversibly dimerizes. It is known to undergo cycloaddition with 1,3-dienes in a reaction similar to the Diels-Alder reaction.

