Stephen aldehyde synthesis
Encyclopedia
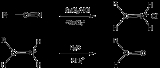
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen
(OBE
/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride
(SnCl2), hydrochloric acid
(HCl) and quenching the resulting iminium
salt ([R-CH=NH2]+Cl-) with water (H2O).
Overall, the reaction scheme is as follows:
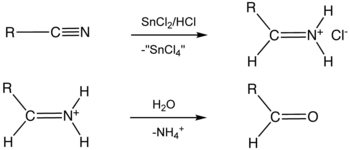
PhCONHPh with phosphorus pentachloride.
Henry Stephen
Henry Stephen OBE was an English chemist who might be best remembered for inventing the Stephen reaction, a way to make aldehydes from nitriles...
(OBE
Order of the British Empire
The Most Excellent Order of the British Empire is an order of chivalry established on 4 June 1917 by George V of the United Kingdom. The Order comprises five classes in civil and military divisions...
/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride
Tin(II) chloride
Tin chloride is a white crystalline solid with the formula 2. It forms a stable dihydrate, but aqueous solutions tend to undergo hydrolysis, particularly if hot. SnCl2 is widely used as a reducing agent , and in electrolytic baths for tin-plating...
(SnCl2), hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
(HCl) and quenching the resulting iminium
Iminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
salt ([R-CH=NH2]+Cl-) with water (H2O).
Overall, the reaction scheme is as follows:
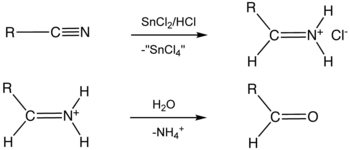
Sonn-Müller method
In the Sonn-Müller method the intermediate iminium salt is obtained from reaction of an amideAmide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
PhCONHPh with phosphorus pentachloride.

