_triflate.gif)
Scandium(III) triflate
Encyclopedia
Scandium trifluoromethanesulfonate, commonly called scandium triflate, is a chemical compound
with formula Sc(SO3CF3)3, a salt consisting of scandium
cations Sc3+ and triflate
SO3CF3− anions.
Scandium triflate is used as a reagent in organic chemistry as a Lewis acid
. Compared to other Lewis acids this reagent is stable towards water and can often be used in an organic reaction
as a true catalyst rather than one used in stoichiometric amounts. The compound is prepared by reaction of scandium oxide with trifluoromethanesulfonic acid
.
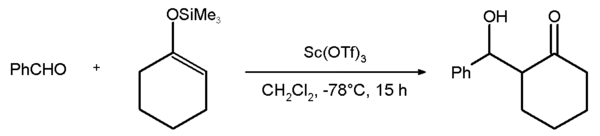
An example of the use of scandium triflate is the Mukaiyama aldol addition
reaction between benzaldehyde
and the silyl enol ether
of cyclohexanone
with an 81% chemical yield.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with formula Sc(SO3CF3)3, a salt consisting of scandium
Scandium
Scandium is a chemical element with symbol Sc and atomic number 21. A silvery-white metallic transition metal, it has historically been sometimes classified as a rare earth element, together with yttrium and the lanthanoids...
cations Sc3+ and triflate
Triflate
Trifluoromethanesulfonate, also known by the trivial name triflate, is a functional group with the formula CF3SO3-. The triflate group is often represented by -OTf, as opposed to -Tf...
SO3CF3− anions.
Scandium triflate is used as a reagent in organic chemistry as a Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
. Compared to other Lewis acids this reagent is stable towards water and can often be used in an organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
as a true catalyst rather than one used in stoichiometric amounts. The compound is prepared by reaction of scandium oxide with trifluoromethanesulfonic acid
Trifluoromethanesulfonic acid
Trifluoromethanesulfonic acid, also known as triflic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification.-Properties:Triflic acid is a hygroscopic, colorless...
.
An example of the use of scandium triflate is the Mukaiyama aldol addition
Mukaiyama aldol addition
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde catalyzed by a Lewis acid. This choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of...
reaction between benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
and the silyl enol ether
Silyl enol ether
Silyl enol ethers in organic chemistry are a class of organic compounds that share a common functional group composed of an enolate bonded through its oxygen terminus to an organosilicon group....
of cyclohexanone
Cyclohexanone
Cyclohexanone is the organic compound with the formula 5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oil has an odor reminiscent of peardrop sweets as well as acetone. Over time, samples assume a yellow color due to oxidation...
with an 81% chemical yield.