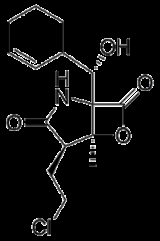
Salinosporamide A
Encyclopedia
Salinosporamide A is a potent proteasome inhibitor
used as an anticancer agent that recently entered phase I human clinical trial
s for the treatment of multiple myeloma
only three years after its discovery. This novel marine natural product is produced by the recently described obligate marine bacterium Salinispora tropica which is found in ocean sediment. Salinosporamide A belongs to a family of compounds possessing a densely functionalized γ-lactam-β-lactone bicycle.
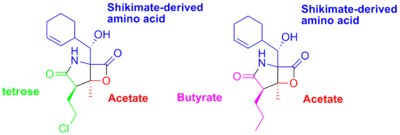
 It was originally hypothesized that Salinosporamide B was a biosynthetic precursor to Salinosporamide A due to their structural similarities.
It was originally hypothesized that Salinosporamide B was a biosynthetic precursor to Salinosporamide A due to their structural similarities.
It was thought that the halogenation of the unactivated methyl group was catalyzed by a non-heme iron halogenase. Recent work using 13C-labeled feeding experiments reveal distinct biosynthetic origins of salinosporamide A and B.
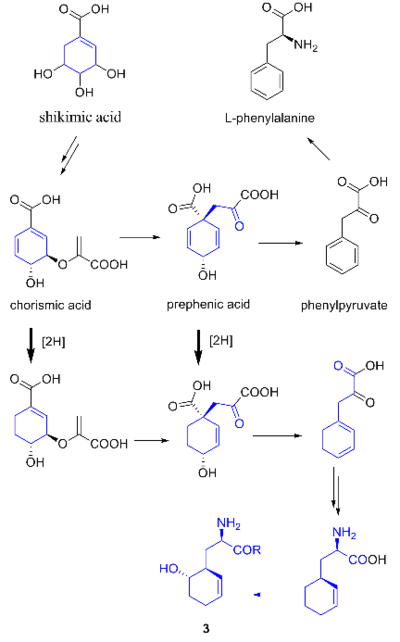
While they share the biosynthetic precursors acetate
and presumed β-hydroxycyclohex-2'-enylalanine (3), they differ in the origin of the four-carbon building block that gives rise to their structural differences involving the halogen
atom. A hybrid polyketide synthase
-nonribosomal peptide synthetase (PKS-NRPS) pathway is most likely the biosynthetic mechanism in which acetyl-CoA
and butyrate-derived ethylmalonyl-CoA condense to yield the β-ketothioester (4), which then reacts with (3) to generate the linear precursor (5).
Later several routes to the total synthesis of Salinosporamide A have been reported.
studies using purified 20S proteasomes showed that Salinosporamide A has lower EC50
for trypsin-like (T-L) activity than does Bortezomib
. In vivo animal model studies show marked inhibition of T-L activity in response to Salinosporamide A, whereas Bortezomib enhances T-L proteasome activity.
Proteasome inhibitor
Proteasome inhibitors are drugs that block the action of proteasomes, cellular complexes that break down proteins, like the p53 protein. Proteasome inhibitors are being studied in the treatment of cancer.-Examples:...
used as an anticancer agent that recently entered phase I human clinical trial
Clinical trial
Clinical trials are a set of procedures in medical research and drug development that are conducted to allow safety and efficacy data to be collected for health interventions...
s for the treatment of multiple myeloma
Multiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
only three years after its discovery. This novel marine natural product is produced by the recently described obligate marine bacterium Salinispora tropica which is found in ocean sediment. Salinosporamide A belongs to a family of compounds possessing a densely functionalized γ-lactam-β-lactone bicycle.
History
Salinosporamide A was discovered by William Fenical and Paul Jensen from Scripps Institution of Oceanography in La Jolla, CA. In preliminary screening, a high percentage of the organic extracts of cultured Salinospora strains possessed antibiotic and anticancer activities, which suggests that these bacteria are an excellent resource for drug discovery. Salinospora strain CNB-392 was isolated from a heat-treated marine sediment sample and cytotoxicity-guided fractionation of the crude extract led to the isolation of salinosporamide A. Although salinosporamide A shares an identical bicyclic ring structure with omuralide, it is uniquely functionalized. Salinosporamide A displayed potent in vitro cytotoxicity against HCT-116 human colon carcinoma with an IC50 value of 11 ng mL-1. This compound also displayed potent and highly selective activity in the NCI's 60-cell-line panel with a mean GI50 value (the concentration required to achieve 50 % growth inhibition) of less than 10 nM and a greater than 4 log LC50 differential between resistant and susceptible cell lines. The greatest potency was observed against NCI-H226 non-small cell lung cancer, SF-539 CNS cancer, SK-MEL-28 melanoma, and MDA-MB-435 breast cancer (all with LC50 values less than 10 nM). Salinosporamide A was tested for its effects on proteasome function because of its structural relationship to omuralide. When tested against purified 20S proteasome, salinosporamide A inhibited proteasomal chymotrypsin-like proteolytic activity with an IC50 value of 1.3 nM. This compound is approximately 35 times more potent than omuralide which was tested as a positive control in the same assay. Thus, the unique functionalization of the core bicyclic ring structure of salinosporamide A appears to have resulted in a molecule that is a significantly more potent proteasome inhibitor than omuralide.Mechanism of action
Salinosporamide A inhibits proteasome activity by covalently modifying the active site threonine residues of the 20S proteasome.Biosynthesis


It was thought that the halogenation of the unactivated methyl group was catalyzed by a non-heme iron halogenase. Recent work using 13C-labeled feeding experiments reveal distinct biosynthetic origins of salinosporamide A and B.
While they share the biosynthetic precursors acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
and presumed β-hydroxycyclohex-2'-enylalanine (3), they differ in the origin of the four-carbon building block that gives rise to their structural differences involving the halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
atom. A hybrid polyketide synthase
Polyketide synthase
Polyketide synthases are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages...
-nonribosomal peptide synthetase (PKS-NRPS) pathway is most likely the biosynthetic mechanism in which acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
and butyrate-derived ethylmalonyl-CoA condense to yield the β-ketothioester (4), which then reacts with (3) to generate the linear precursor (5).
Total synthesis
First stereoselective synthesis was reported by Rajender Reddy Leleti and E. J.Corey.Later several routes to the total synthesis of Salinosporamide A have been reported.
Clinical use
In vitroIn vitro
In vitro refers to studies in experimental biology that are conducted using components of an organism that have been isolated from their usual biological context in order to permit a more detailed or more convenient analysis than can be done with whole organisms. Colloquially, these experiments...
studies using purified 20S proteasomes showed that Salinosporamide A has lower EC50
EC50
The term half maximal effective concentration refers to the concentration of a drug, antibody or toxicant which induces a response halfway between the baseline and maximum after some specified exposure time...
for trypsin-like (T-L) activity than does Bortezomib
Bortezomib
Bortezomib is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma...
. In vivo animal model studies show marked inhibition of T-L activity in response to Salinosporamide A, whereas Bortezomib enhances T-L proteasome activity.

