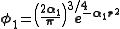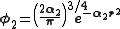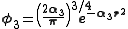STO-nG basis sets
Encyclopedia
STO-nG basis sets are minimal basis sets
, where primitive Gaussian orbital
primitive Gaussian orbital
s are fitted to a single Slater-type orbital
(STO). originally took the values 2 - 6. They were first proposed by John Pople
originally took the values 2 - 6. They were first proposed by John Pople
. A minimum basis set is where only sufficient orbitals are used to contain all the electrons in the neutral atom. Thus for the hydrogen atom, only a single 1s orbital is needed, while for a carbon atoms, 1s, 2s and three 2p orbitals are needed. The core and valence orbitals are represented by the same number of primitive Gaussian functions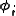 . For example, an STO-3G basis set for the 1s, 2s and 2p orbital of the carbon atom are all linear combination of 3 primitive Gaussian functions. For example, a STO-3G s orbital is given by:
. For example, an STO-3G basis set for the 1s, 2s and 2p orbital of the carbon atom are all linear combination of 3 primitive Gaussian functions. For example, a STO-3G s orbital is given by:
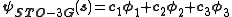
where
The values of c1, c2, c3, α1, α2 and α3 have to be determined. For the STO-nG basis sets, they are obtained by making a least squares
fit of the three Gaussian orbitals to the single Slater-type orbitals. This differs from the more common procedure where the criteria often used is to choose the coefficients (c's) and exponents (α's) to give the lowest energy with some appropriate method for some appropriate molecule. A special feature of this basis set is that common exponents are used for orbitals in the same shell (e.g. 2s and 2p) as this allows more efficient computation.
The fit between the Gaussian orbitals and the Slater orbital is good for all values of r, except for very small values near to the nucleus. The Slater orbital has a cusp at the nucleus, while Gaussian orbitals are flat at the nucleus.
Basis set (chemistry)
A basis set in chemistry is a set of functions used to create the molecular orbitals, which are expanded as a linear combination of such functions with the weights or coefficients to be determined. Usually these functions are atomic orbitals, in that they are centered on atoms. Otherwise, the...
, where
 primitive Gaussian orbital
primitive Gaussian orbitalGaussian orbital
In computational chemistry and molecular physics, Gaussian orbitals are functions used as atomic orbitals in the LCAO method for the computation of electron orbitals in molecules and numerous properties that depend on these.- Rationale :The principal reason for the use of Gaussian basis functions...
s are fitted to a single Slater-type orbital
Slater-type orbital
Slater-type orbitals are functions used as atomic orbitals in the linear combination of atomic orbitals molecular orbital method. They are named after the physicist John C. Slater, who introduced them in 1930....
(STO).
 originally took the values 2 - 6. They were first proposed by John Pople
originally took the values 2 - 6. They were first proposed by John PopleJohn Pople
Sir John Anthony Pople, KBE, FRS, was a Nobel-Prize winning theoretical chemist. Born in Burnham-on-Sea, Somerset, England, he attended Bristol Grammar School. He won a scholarship to Trinity College, Cambridge in 1943. He received his B. A. in 1946. Between 1945 and 1947 he worked at the Bristol...
. A minimum basis set is where only sufficient orbitals are used to contain all the electrons in the neutral atom. Thus for the hydrogen atom, only a single 1s orbital is needed, while for a carbon atoms, 1s, 2s and three 2p orbitals are needed. The core and valence orbitals are represented by the same number of primitive Gaussian functions
 . For example, an STO-3G basis set for the 1s, 2s and 2p orbital of the carbon atom are all linear combination of 3 primitive Gaussian functions. For example, a STO-3G s orbital is given by:
. For example, an STO-3G basis set for the 1s, 2s and 2p orbital of the carbon atom are all linear combination of 3 primitive Gaussian functions. For example, a STO-3G s orbital is given by: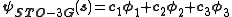
where
The values of c1, c2, c3, α1, α2 and α3 have to be determined. For the STO-nG basis sets, they are obtained by making a least squares
Least squares
The method of least squares is a standard approach to the approximate solution of overdetermined systems, i.e., sets of equations in which there are more equations than unknowns. "Least squares" means that the overall solution minimizes the sum of the squares of the errors made in solving every...
fit of the three Gaussian orbitals to the single Slater-type orbitals. This differs from the more common procedure where the criteria often used is to choose the coefficients (c's) and exponents (α's) to give the lowest energy with some appropriate method for some appropriate molecule. A special feature of this basis set is that common exponents are used for orbitals in the same shell (e.g. 2s and 2p) as this allows more efficient computation.
The fit between the Gaussian orbitals and the Slater orbital is good for all values of r, except for very small values near to the nucleus. The Slater orbital has a cusp at the nucleus, while Gaussian orbitals are flat at the nucleus.
Use of STO-nG basis sets
The most widely used basis set of this group is STO-3G, which is used for large systems and for preliminary geometry determinations. This basis set is available for all atoms from Hydrogen up to Xenon.STO-2G basis set
The STO-2G basis set is a linear combination of 2 primitive Gaussian functions. The original coefficients and exponents for first-row and second-row atoms are given as follows.| STO-2G | α1 | c1 | α2 | c2 |
| 1s | 0.151623 | 0.678914 | 0.851819 | 0.430129 |
| 2s | 0.0974545 | 0.963782 | 0.384244 | 0.0494718 |
| 2p | 0.0974545 | 0.61282 | 0.384244 | 0.511541 |
Accuracy
The exact energy of the 1s electron of H atom is -0.5 hartree, given by a single Slater-type orbital with exponent 1.0. The following table illustrates the increase in accuracy as the number of primitive Gaussian functions increases from 3 to 6 in the basis set.| Basis set | Energy [hartree] |
| STO-3G | -0.49491 |
| STO-4G | -0.49848 |
| STO-5G | -0.49951 |
| STO-6G | -0.49983 |