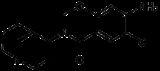
Renzapride
Encyclopedia
Renzapride is a gastroprokinetic agent and antiemetic
which acts as a full 5-HT4 full agonist and 5-HT3 antagonist
. It also functions as a 5-HT2B
antagonist and has some affinity for the 5-HT2A and 5-HT2C receptors, though it is unlikely that these properties contribute to its therapeutic effects.
Renzapride was being developed by Alizyme plc of the United Kingdom.
(IBS-C). It is also potentially effective for irritable bowel syndrome
with alternating stool pattern (IBS-A). It is being developed by Alizyme plc of the United Kingdom.
As of 23 April 2008, Alizyme ceased all development of renzapride, after a Phase III trial in the U.S. did not show enough efficacy over placebo to justify further development.
Antiemetic
An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer....
which acts as a full 5-HT4 full agonist and 5-HT3 antagonist
Receptor antagonist
A receptor antagonist is a type of receptor ligand or drug that does not provoke a biological response itself upon binding to a receptor, but blocks or dampens agonist-mediated responses...
. It also functions as a 5-HT2B
5-HT2B receptor
5-hydroxytryptamine receptor 2B, also known as HTR2B, is a 5-HT2 receptor, but also denotes the human gene encoding it.-Function:...
antagonist and has some affinity for the 5-HT2A and 5-HT2C receptors, though it is unlikely that these properties contribute to its therapeutic effects.
Renzapride was being developed by Alizyme plc of the United Kingdom.
Clinical trials
Renzapride was being investigated for the treatment of constipation-predominant irritable bowel syndromeIrritable bowel syndrome
Irritable bowel syndrome is a diagnosis of exclusion. It is a functional bowel disorder characterized by chronic abdominal pain, discomfort, bloating, and alteration of bowel habits in the absence of any detectable organic cause. In some cases, the symptoms are relieved by bowel movements...
(IBS-C). It is also potentially effective for irritable bowel syndrome
Irritable bowel syndrome
Irritable bowel syndrome is a diagnosis of exclusion. It is a functional bowel disorder characterized by chronic abdominal pain, discomfort, bloating, and alteration of bowel habits in the absence of any detectable organic cause. In some cases, the symptoms are relieved by bowel movements...
with alternating stool pattern (IBS-A). It is being developed by Alizyme plc of the United Kingdom.
As of 23 April 2008, Alizyme ceased all development of renzapride, after a Phase III trial in the U.S. did not show enough efficacy over placebo to justify further development.

