Prismane
Encyclopedia
Prismane is a polycyclic hydrocarbon
with the formula
C6H6. It is an isomer
of benzene
, more specific: a valence isomer
. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule
are arranged in the shape of a six-atom triangular prism
. Albert Ladenburg
proposed this structure for the compound now known as benzene
. The compound was not synthesized until 1973.
. The first, proposed by Kekulé
in 1867, later proved to be closest to the true structure of benzene. This structure inspired several others to come up with concurring structures; for example,
Ladenburg
with prismane, Dewar
with Dewar benzene
, and Koerner and Claus with Claus' benzene
. Some of these structures could be synthesized in the following years. Prismane, like the other proposed structures for benzene, is still often cited in the literature, because it is part of the historical struggle toward understanding the mesomeric structures and resonance of benzene. Some computational chemists still research the differences among the possible isomers of C6H6.
leads to a high ring strain
, reminiscent of that of cyclopropane
but greater. The compound is explosive, which is unusual for a hydrocarbon. Due to this ring strain, the bonds have a low bond energy and break at a low activation energy
, which makes synthesis of the molecule difficult. The molecule in which all six hydrogens are substituted by methyl groups (hexamethylprismane) has a higher stability and was synthesized by rearrangement reaction
s in 1966.
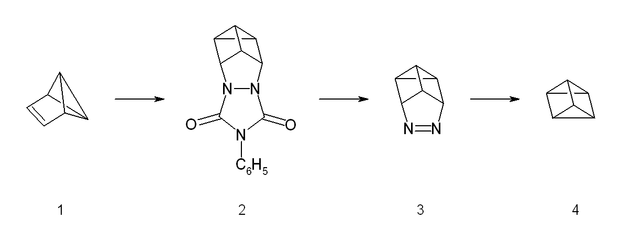 The synthesis starts from benzvalene
The synthesis starts from benzvalene
(1) and 4-phenyltriazolidone
, which is a strong dienophile. The reaction is a stepwise Diels-Alder like reaction
, forming a carbocation
as intermediate. The adduct (2) is then hydrolyzed under basic conditions and afterwards transformed into a copper(II) chloride
derivative with acidic copper(II) chloride. Neutralized with a strong base, the azo
compound (3) could be crystallized with 65% yield. The last step is a photolysis of the azo compound. This photolysis leads to a biradical which forms prismane (4) and nitrogen
with a yield of less than 10%. The compound was isolated by preparative gas chromatography.
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
C6H6. It is an isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, more specific: a valence isomer
Valence isomer
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions.-Benzene:...
. Prismane is far less stable than benzene. The carbon (and hydrogen) atoms of the prismane molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
are arranged in the shape of a six-atom triangular prism
Prism (geometry)
In geometry, a prism is a polyhedron with an n-sided polygonal base, a translated copy , and n other faces joining corresponding sides of the two bases. All cross-sections parallel to the base faces are the same. Prisms are named for their base, so a prism with a pentagonal base is called a...
. Albert Ladenburg
Albert Ladenburg
Albert Ladenburg was a German chemist.-Biography:Ladenburg was a member of a well known Jewish family in Mannheim. He was educated at a Realgymnasium at Mannheim and then, after the age of 15, at the technical school of Karlsruhe, where he studied mathematics and modern languages...
proposed this structure for the compound now known as benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
. The compound was not synthesized until 1973.
History
Discussion about the structure of benzene in the mid 19th century yielded several structures for the formula C6H6, which was accessible by combustion analysisCombustion analysis
Combustion analysis is a method used in both organic chemistry and analytical chemistry to determine the elemental composition of a pure organic compound by combusting the sample under conditions where the resulting combustion products can be quantitatively analyzed...
. The first, proposed by Kekulé
Friedrich August Kekulé von Stradonitz
Friedrich August Kekule von Stradonitz was a German organic chemist. From the 1850s until his death, Kekule was one of the most prominent chemists in Europe, especially in theoretical chemistry...
in 1867, later proved to be closest to the true structure of benzene. This structure inspired several others to come up with concurring structures; for example,
Ladenburg
Albert Ladenburg
Albert Ladenburg was a German chemist.-Biography:Ladenburg was a member of a well known Jewish family in Mannheim. He was educated at a Realgymnasium at Mannheim and then, after the age of 15, at the technical school of Karlsruhe, where he studied mathematics and modern languages...
with prismane, Dewar
James Dewar
Sir James Dewar FRS was a Scottish chemist and physicist. He is probably best-known today for his invention of the Dewar flask, which he used in conjunction with extensive research into the liquefaction of gases...
with Dewar benzene
Dewar benzene
Dewar benzene or bicyclo[2.2.0]hexa-2,5-diene is a bicyclic isomer of benzene with the molecular formula C6H6. The compound is named after James Dewar who included this structure in a list of possible C6H6 structures in 1867....
, and Koerner and Claus with Claus' benzene
Claus' benzene
Claus' benzene is a hypothetical hydrocarbon and an isomer of benzene . It was proposed by Adolf Karl Ludwig Claus in 1867 as a possible structure for benzene at a time when the structure of benzene was debated...
. Some of these structures could be synthesized in the following years. Prismane, like the other proposed structures for benzene, is still often cited in the literature, because it is part of the historical struggle toward understanding the mesomeric structures and resonance of benzene. Some computational chemists still research the differences among the possible isomers of C6H6.
Properties
It is a colourless liquid. The deviation of the carbon-carbon bond angle from 109° to 60° in a triangleTriangle
A triangle is one of the basic shapes of geometry: a polygon with three corners or vertices and three sides or edges which are line segments. A triangle with vertices A, B, and C is denoted ....
leads to a high ring strain
Ring strain
In organic chemistry, ring strain is the tendency of a cyclic molecule, such as cyclopropane, to destabilize when its atoms are in non-favorable high energy spatial orientations...
, reminiscent of that of cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
but greater. The compound is explosive, which is unusual for a hydrocarbon. Due to this ring strain, the bonds have a low bond energy and break at a low activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
, which makes synthesis of the molecule difficult. The molecule in which all six hydrogens are substituted by methyl groups (hexamethylprismane) has a higher stability and was synthesized by rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s in 1966.
Synthesis

Benzvalene
Benzvalene is an organic compound and one of several isomers of benzene.. It was synthesised in 1971 by Thomas J. Katz et al.The 1971 synthesis consisted of treating cyclopentadiene with methyllithium in dimethylether and then with dichloromethane and methyllithium in at -45°C. The hydrocarbon in...
(1) and 4-phenyltriazolidone
4-Phenyl-1,2,4-triazole-3,5-dione
4-Phenyl-1,2,4-triazole-3,5-dione is an azodicarbonyl compound. PTAD is one of the strongest dienophiles and reacts rapidly with dienes in Diels-Alder reactions. The most prominent use of PTAD was the first successful synthesis of prismane in 1973....
, which is a strong dienophile. The reaction is a stepwise Diels-Alder like reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
, forming a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
as intermediate. The adduct (2) is then hydrolyzed under basic conditions and afterwards transformed into a copper(II) chloride
Copper(II) chloride
Copper chloride is the chemical compound with the formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. The copper chlorides are some of the most common copper compounds, after copper sulfate....
derivative with acidic copper(II) chloride. Neutralized with a strong base, the azo
Azo
Azo may refer to:* Azo compound, chemistry functional group and class of compounds, also used for CDs and DVDs* A urinary tract analgesic also known as phenazopyridine* the medieval Italian jurist, see Azo of Bologna...
compound (3) could be crystallized with 65% yield. The last step is a photolysis of the azo compound. This photolysis leads to a biradical which forms prismane (4) and nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
with a yield of less than 10%. The compound was isolated by preparative gas chromatography.
See also
- Prismane C8, C8
- CubaneCubaneCubane is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons. It was first synthesized in 1964 by Philip Eaton, a...
, C8H8 - FullereneFullereneA fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...

