
Plutonium hexafluoride
Encyclopedia
Plutonium hexafluoride is the highest fluoride of plutonium
, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239
from irradiated uranium. This pure plutonium is needed to avoid premature ignition of low-mass nuclear weapon
designs by neutrons produced by spontaneous fission of plutonium-240
.
It is a red-brown volatile crystalline solid; the heat of sublimation is 12.1 kcal/mol and the heat of vaporization 7.4 kcal/mol. It is relatively hard to handle, being very corrosive and prone to auto-radiolysis
.
It is prepared by fluorination of plutonium tetrafluoride (PuF4) by powerful fluorinating agents such as elemental fluorine.
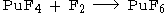
Its further be obtained by fluorination of plutonium(III) fluoride
or plutonium(IV) oxide.
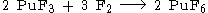
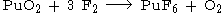
Hydrogen fluoride
is not sufficient; it is itself a powerful fluorinating agent.
Under laser irradiation at a wavelength of less than 520 nm, it decomposes to plutonium pentafluoride and fluorine
; after more irradiation it decomposes further to plutonium tetrafluoride.
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
, and is of interest for laser enrichment of plutonium, in particular for the production of pure plutonium-239
Plutonium-239
Plutonium-239 is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in...
from irradiated uranium. This pure plutonium is needed to avoid premature ignition of low-mass nuclear weapon
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
designs by neutrons produced by spontaneous fission of plutonium-240
Plutonium-240
Plutonium-240 is an isotope of the metal plutonium formed when plutonium-239 captures a neutron. About 62% to 73% of the time when Pu-239 captures a neutron it undergoes fission; the rest of the time it forms Pu-240. The longer a nuclear fuel element remains in a nuclear reactor the greater the...
.
It is a red-brown volatile crystalline solid; the heat of sublimation is 12.1 kcal/mol and the heat of vaporization 7.4 kcal/mol. It is relatively hard to handle, being very corrosive and prone to auto-radiolysis
Radiolysis
Radiolysis is the dissociation of molecules by nuclear radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux...
.
It is prepared by fluorination of plutonium tetrafluoride (PuF4) by powerful fluorinating agents such as elemental fluorine.
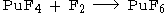
Its further be obtained by fluorination of plutonium(III) fluoride
Plutonium(III) fluoride
Plutonium fluoride or plutonium trifluoride is the chemical compound composed of plutonium and fluorine with the formula PuF3. It forms violet crystals...
or plutonium(IV) oxide.
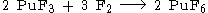
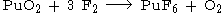
Hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
is not sufficient; it is itself a powerful fluorinating agent.
Under laser irradiation at a wavelength of less than 520 nm, it decomposes to plutonium pentafluoride and fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
; after more irradiation it decomposes further to plutonium tetrafluoride.

