
Plutonium-239
Encyclopedia
Plutonium-239 is an isotope
of plutonium
. Plutonium-239 is the primary fissile
isotope used for the production of nuclear weapon
s, although uranium-235
has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in nuclear reactor
s, along with uranium-235
and uranium-233
. Plutonium-239 has a half-life
of 24,200 years.
of an atom of uranium-235 in the reactor of a nuclear power plant
produces two to three neutrons, and these neutrons can be absorbed by uranium-238 to produce plutonium-239 and other isotope
s. Plutonium-239 can also absorb neutrons and fission along with the uranium-235 in a reactor.
Of all the common nuclear fuels, Pu-239 has the smallest critical mass.
A spherical untampered critical mass is about 11 kg (24.2 lbs), 10.2 cm (4") in diameter. Using appropriate triggers, neutron reflectors, implosion geometry and tampers, this critical mass can be reduced by more than twofold. This optimization usually requires a large nuclear development organization supported by a sovereign nation.
The fission of one atom of Pu-239 generates 207.1 MeV
= 3.318 × 10−11 J, i.e. 19.98 TJ/mol
= 83.61 TJ/kg.
is exposed to neutron radiation
, its nucleus will capture a neutron
, changing it to U-239. This happens more easily with lower Kinetic Energy (as U-238 fission activation is 6.6MeV). The U-239 then rapidly undergoes two beta decay
s. After the 238U absorbs a neutron to become 239U it then emits an electron
and an anti-neutrino (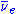 ) by β− decay
) by β− decay
to become Neptunium-239 (239Np) and then emits another electron and anti-neutrino by a second β− decay to become 239Pu:
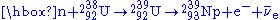
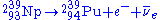
Fission activity is relatively rare, so even after significant exposure, the Pu-239 is still mixed with a great deal of U-238 (and possibly other isotopes of uranium), oxygen, other components of the original material, and fission products. Only if the fuel has been exposed for a few days in the reactor, can the Pu-239 be chemically separated
from the rest of the material to yield high-purity Pu-239 metal.
Pu-239 has a higher probability for fission than U-235 and a larger number of neutrons produced per fission event, so it has a smaller critical mass
. Pure Pu-239 also has a reasonably low rate of neutron emission due to spontaneous fission
(10 fission/s-kg), making it feasible to assemble a mass that is highly supercritical before a detonation chain reaction
begins.
In practice, however, reactor-bred plutonium produced will invariably contain a certain amount of Pu-240 due to the tendency of Pu-239 to absorb an additional neutron during production. Pu-240 has a high rate of spontaneous fission
events (415,000 fission/s-kg), making it an undesirable contaminant. As a result, plutonium containing a significant fraction of Pu-240 is not well-suited to use in nuclear weapons; it emits neutron radiation
, making handling more difficult, and its presence can lead to a "fizzle" in which a small explosion occurs, destroying the weapon but not causing fission of a significant fraction of the fuel. (However, in modern nuclear weapons using neutron generators for initiation and fusion boosting to supply extra neutrons, fizzling may not be an issue.) It is because of this limitation that plutonium-based weapons must be implosion-type, rather than gun-type. (The US has constructed a single experimental bomb using only reactor-grade plutonium.) Moreover, Pu-239 and Pu-240 cannot be chemically distinguished, so expensive and difficult isotope separation
would be necessary to separate them. Weapons-grade plutonium is defined as containing no more than 7% Pu-240; this is achieved by only exposing U-238 to neutron sources for short periods of time to minimize the Pu-240 produced. Pu-240 exposed to alpha particles will incite a nuclear fission.
Plutonium is classified according to the percentage of the contaminant plutonium-240 that it contains:
A nuclear reactor that is used to produce plutonium for weapons therefore generally has a means for exposing U-238 to neutron radiation and for frequently replacing the irradiated U-238 with new U-238. A reactor running on unenriched or moderately enriched uranium contains a great deal of U-238. However, most commercial nuclear power reactor designs require the entire reactor to shut down, often for weeks, in order to change the fuel elements. They therefore produce plutonium in a mix of isotopes that is not well-suited to weapon construction. Such a reactor could have machinery added that would permit U-238 slugs to be placed near the core and changed frequently, or it could be shut down frequently, so proliferation is a concern; for this reason, the International Atomic Energy Agency
inspects licensed reactors often. A few commercial power reactor designs, such as the reaktor bolshoy moshchnosti kanalniy (RBMK
) and pressurized heavy water reactor (PHWR), do permit refueling without shutdowns, and they may pose a proliferation risk. (In fact, the RBMK
was built by the Soviet Union during the cold war, so despite their ostensibly peaceful purpose, it is likely that plutonium production was a design criterion.) By contrast, the Canadian CANDU heavy-water moderated natural-uranium fueled reactor can also be refueled while operating, but it normally consumes most of the Pu-239 it produces in situ; thus, it is not only inherently less proliferative than most reactors, but can even be operated as an "actinide incinerator." The American IFR
(Integral Fast Reactor) can also be operated in an "incineration mode," having some advantages in not building up the Pu-242 isotope or the long-lived actinide
s, either of which cannot be easily burned except in a fast reactor. Also IFR fuel has a high proportion of burnable isotopes, while in CANDU an inert material is needed to dilute the fuel; this means the IFR can burn a higher fraction of its fuel before needing reprocessing.
Most plutonium is produced in research reactor
s or plutonium production reactors called breeder reactor
s because they produce more plutonium than they consume fuel; in principle, such reactors make extremely efficient use of natural uranium. In practice, their construction and operation is sufficiently difficult that they are generally only used to produce plutonium. Breeder reactors are generally (but not always) fast reactors, since fast neutrons are somewhat more efficient at plutonium production.
s in place of the conventional plutonium
used in the Air Force's versions. "Supergrade" is industry parlance for plutonium alloy bearing an exceptionally high fraction of Pu-239 (>95%), leaving a very low amount of Pu-240 which is a high spontaneous fission
isotope
(see above). Such plutonium is produced from fuel rods that have been irradiated a very short time as measured in MW-Day/Ton burnup
. Such low irradiation times limit the amount of additional neutron capture
and therefore buildup of alternate isotope products such as Pu-240 in the rod, and also by consequence is considerably more expensive to produce, needing far more rods irradiated and processed for a given amount of plutonium.
Plutonium-240, in addition to being a neutron emitter after fission, is a gamma emitter in that process as well, and so is responsible for a large fraction of the radiation from stored nuclear weapons. Submarine
crew members routinely operate in close proximity to stored weapons in torpedo rooms, unlike Air Force
missiles where exposures are relatively brief - hence justifying the additional costs of the premium supergrade alloy used on many naval nuclear torpedo weapons. Supergrade plutonium is used in W80 warheads.
that allows a significant amount of plutonium to build up in irradiated reactor fuel. Plutonium-239 will be present both in the reactor core during operation and in spent nuclear fuel
that has been removed from the reactor at the end of the fuel assembly’s service life (typically several years). Spent nuclear fuel commonly contains about 0.8% plutonium-239.
Plutonium-239 present in reactor fuel can absorb neutrons and fission just as uranium-235 can. Since plutonium-239 is constantly being created in the reactor core during operation, the use of plutonium-239 as nuclear fuel in power plants can occur without reprocessing of spent fuel
; the plutonium-239 is fissioned in the same fuel rods in which it is produced. Fissioning of plutonium-239 provides about one-third of the total energy produced in a typical commercial nuclear power plant. Reactor fuel would accumulate much more than 0.8% plutonium-239 during its service life if some plutonium-239 were not constantly being “burned off” by fissioning.
A small percentage of plutonium-239 can be deliberately added to fresh nuclear fuel. Such fuel is called MOX (mixed oxide) fuel
, as it contains a mixture of uranium oxide (UO2) and plutonium oxide (PuO2). The addition of plutonium-239 reduces or eliminates the need to enrich the uranium
in the fuel.
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
. Plutonium-239 is the primary fissile
Fissile
In nuclear engineering, a fissile material is one that is capable of sustaining a chain reaction of nuclear fission. By definition, fissile materials can sustain a chain reaction with neutrons of any energy. The predominant neutron energy may be typified by either slow neutrons or fast neutrons...
isotope used for the production of nuclear weapon
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s, although uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
has also been used and is currently the secondary isotope. Plutonium-239 is also one of the three main isotopes demonstrated usable as fuel in nuclear reactor
Nuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s, along with uranium-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
and uranium-233
Uranium-233
Uranium-233 is a fissile isotope of uranium, bred from Thorium as part of the thorium fuel cycle. It has been used in a few nuclear reactors and has been proposed for much wider use as a nuclear fuel. It has a half-life of 160,000 years....
. Plutonium-239 has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 24,200 years.
Nuclear properties
The nuclear properties of plutonium-239, as well as the ability to produce large amounts of nearly pure Pu-239, led to its use in nuclear weapons and nuclear power stations. The fissioningNuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
of an atom of uranium-235 in the reactor of a nuclear power plant
Nuclear power plant
A nuclear power plant is a thermal power station in which the heat source is one or more nuclear reactors. As in a conventional thermal power station the heat is used to generate steam which drives a steam turbine connected to a generator which produces electricity.Nuclear power plants are usually...
produces two to three neutrons, and these neutrons can be absorbed by uranium-238 to produce plutonium-239 and other isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s. Plutonium-239 can also absorb neutrons and fission along with the uranium-235 in a reactor.
Of all the common nuclear fuels, Pu-239 has the smallest critical mass.
A spherical untampered critical mass is about 11 kg (24.2 lbs), 10.2 cm (4") in diameter. Using appropriate triggers, neutron reflectors, implosion geometry and tampers, this critical mass can be reduced by more than twofold. This optimization usually requires a large nuclear development organization supported by a sovereign nation.
The fission of one atom of Pu-239 generates 207.1 MeV
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
= 3.318 × 10−11 J, i.e. 19.98 TJ/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
= 83.61 TJ/kg.
| type of radiation source (fission of Pu-239) | Average energy released [MeV] |
|---|---|
| Instantaneously released energy | |
| Kinetic energy of fission fragments | 175.8 |
| Kinetic energy of prompt neutrons | 5.9 |
| Energy carried by prompt γ-rays | 7.8 |
| Energy from decaying fission products | |
| Energy of β−-particles | 5.3 |
| Energy of anti-neutrinos | 7.1 |
| Energy of delayed γ-rays | 5.2 |
| Sum (total decay energy) | 207.1 |
| Energy released when those prompt neutrons which don't (re)produce fission are captured | 11.5 |
| Energy converted into heat in an operating thermal nuclear reactor (antineutrino energy escapes reactor and does not appear in total heat) | 211.5 |
Manufacturing
Pu-239 is normally created in nuclear reactors by transmutation of individual atoms of one of the isotopes of uranium present in the fuel rods. Occasionally, when an atom of U-238Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
is exposed to neutron radiation
Neutron radiation
Neutron radiation is a kind of ionizing radiation which consists of free neutrons. A result of nuclear fission or nuclear fusion, it consists of the release of free neutrons from atoms, and these free neutrons react with nuclei of other atoms to form new isotopes, which, in turn, may produce...
, its nucleus will capture a neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
, changing it to U-239. This happens more easily with lower Kinetic Energy (as U-238 fission activation is 6.6MeV). The U-239 then rapidly undergoes two beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
s. After the 238U absorbs a neutron to become 239U it then emits an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
and an anti-neutrino (
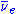 ) by β− decay
) by β− decayBeta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
to become Neptunium-239 (239Np) and then emits another electron and anti-neutrino by a second β− decay to become 239Pu:
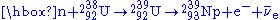
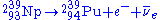
Fission activity is relatively rare, so even after significant exposure, the Pu-239 is still mixed with a great deal of U-238 (and possibly other isotopes of uranium), oxygen, other components of the original material, and fission products. Only if the fuel has been exposed for a few days in the reactor, can the Pu-239 be chemically separated
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
from the rest of the material to yield high-purity Pu-239 metal.
Pu-239 has a higher probability for fission than U-235 and a larger number of neutrons produced per fission event, so it has a smaller critical mass
Critical mass
A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The critical mass of a fissionable material depends upon its nuclear properties A critical mass is the smallest amount of fissile material needed for a sustained nuclear chain reaction. The...
. Pure Pu-239 also has a reasonably low rate of neutron emission due to spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
(10 fission/s-kg), making it feasible to assemble a mass that is highly supercritical before a detonation chain reaction
Nuclear chain reaction
A nuclear chain reaction occurs when one nuclear reaction causes an average of one or more nuclear reactions, thus leading to a self-propagating number of these reactions. The specific nuclear reaction may be the fission of heavy isotopes or the fusion of light isotopes...
begins.
In practice, however, reactor-bred plutonium produced will invariably contain a certain amount of Pu-240 due to the tendency of Pu-239 to absorb an additional neutron during production. Pu-240 has a high rate of spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
events (415,000 fission/s-kg), making it an undesirable contaminant. As a result, plutonium containing a significant fraction of Pu-240 is not well-suited to use in nuclear weapons; it emits neutron radiation
Neutron radiation
Neutron radiation is a kind of ionizing radiation which consists of free neutrons. A result of nuclear fission or nuclear fusion, it consists of the release of free neutrons from atoms, and these free neutrons react with nuclei of other atoms to form new isotopes, which, in turn, may produce...
, making handling more difficult, and its presence can lead to a "fizzle" in which a small explosion occurs, destroying the weapon but not causing fission of a significant fraction of the fuel. (However, in modern nuclear weapons using neutron generators for initiation and fusion boosting to supply extra neutrons, fizzling may not be an issue.) It is because of this limitation that plutonium-based weapons must be implosion-type, rather than gun-type. (The US has constructed a single experimental bomb using only reactor-grade plutonium.) Moreover, Pu-239 and Pu-240 cannot be chemically distinguished, so expensive and difficult isotope separation
Isotope separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes, for example separating natural uranium into enriched uranium and depleted uranium. This is a crucial process in the manufacture of uranium fuel for nuclear power stations, and is...
would be necessary to separate them. Weapons-grade plutonium is defined as containing no more than 7% Pu-240; this is achieved by only exposing U-238 to neutron sources for short periods of time to minimize the Pu-240 produced. Pu-240 exposed to alpha particles will incite a nuclear fission.
Plutonium is classified according to the percentage of the contaminant plutonium-240 that it contains:
- Supergrade 2-3%
- Weapons grade less than 7%
- Fuel grade 7-18%
- Reactor grade 18% or more.
A nuclear reactor that is used to produce plutonium for weapons therefore generally has a means for exposing U-238 to neutron radiation and for frequently replacing the irradiated U-238 with new U-238. A reactor running on unenriched or moderately enriched uranium contains a great deal of U-238. However, most commercial nuclear power reactor designs require the entire reactor to shut down, often for weeks, in order to change the fuel elements. They therefore produce plutonium in a mix of isotopes that is not well-suited to weapon construction. Such a reactor could have machinery added that would permit U-238 slugs to be placed near the core and changed frequently, or it could be shut down frequently, so proliferation is a concern; for this reason, the International Atomic Energy Agency
International Atomic Energy Agency
The International Atomic Energy Agency is an international organization that seeks to promote the peaceful use of nuclear energy, and to inhibit its use for any military purpose, including nuclear weapons. The IAEA was established as an autonomous organization on 29 July 1957...
inspects licensed reactors often. A few commercial power reactor designs, such as the reaktor bolshoy moshchnosti kanalniy (RBMK
RBMK
RBMK is an initialism for the Russian reaktor bolshoy moshchnosti kanalniy which means "High Power Channel-type Reactor", and describes a class of graphite-moderated nuclear power reactor which was built in the Soviet Union. The RBMK reactor was the type involved in the Chernobyl disaster...
) and pressurized heavy water reactor (PHWR), do permit refueling without shutdowns, and they may pose a proliferation risk. (In fact, the RBMK
RBMK
RBMK is an initialism for the Russian reaktor bolshoy moshchnosti kanalniy which means "High Power Channel-type Reactor", and describes a class of graphite-moderated nuclear power reactor which was built in the Soviet Union. The RBMK reactor was the type involved in the Chernobyl disaster...
was built by the Soviet Union during the cold war, so despite their ostensibly peaceful purpose, it is likely that plutonium production was a design criterion.) By contrast, the Canadian CANDU heavy-water moderated natural-uranium fueled reactor can also be refueled while operating, but it normally consumes most of the Pu-239 it produces in situ; thus, it is not only inherently less proliferative than most reactors, but can even be operated as an "actinide incinerator." The American IFR
Integral Fast Reactor
The Integral Fast Reactor is a design for a nuclear reactor using fast neutrons and no neutron moderator . IFR is distinguished by a nuclear fuel cycle that uses reprocessing via electrorefining at the reactor site.The U.S...
(Integral Fast Reactor) can also be operated in an "incineration mode," having some advantages in not building up the Pu-242 isotope or the long-lived actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s, either of which cannot be easily burned except in a fast reactor. Also IFR fuel has a high proportion of burnable isotopes, while in CANDU an inert material is needed to dilute the fuel; this means the IFR can burn a higher fraction of its fuel before needing reprocessing.
Most plutonium is produced in research reactor
Research reactor
Research reactors are nuclear reactors that serve primarily as a neutron source. They are also called non-power reactors, in contrast to power reactors that are used for electricity production, heat generation, or maritime propulsion.-Purpose:...
s or plutonium production reactors called breeder reactor
Breeder reactor
A breeder reactor is a nuclear reactor capable of generating more fissile material than it consumes because its neutron economy is high enough to breed fissile from fertile material like uranium-238 or thorium-232. Breeders were at first considered superior because of their superior fuel economy...
s because they produce more plutonium than they consume fuel; in principle, such reactors make extremely efficient use of natural uranium. In practice, their construction and operation is sufficiently difficult that they are generally only used to produce plutonium. Breeder reactors are generally (but not always) fast reactors, since fast neutrons are somewhat more efficient at plutonium production.
Supergrade plutonium
The "supergrade" fission fuel, which has less radioactivity, is used in the primary stage of US Navy nuclear weaponNuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s in place of the conventional plutonium
Plutonium
Plutonium is a transuranic radioactive chemical element with the chemical symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, forming a dull coating when oxidized. The element normally exhibits six allotropes and four oxidation...
used in the Air Force's versions. "Supergrade" is industry parlance for plutonium alloy bearing an exceptionally high fraction of Pu-239 (>95%), leaving a very low amount of Pu-240 which is a high spontaneous fission
Spontaneous fission
Spontaneous fission is a form of radioactive decay characteristic of very heavy isotopes. Because the nuclear binding energy reaches a maximum at a nuclear mass greater than about 60 atomic mass units , spontaneous breakdown into smaller nuclei and single particles becomes possible at heavier masses...
isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
(see above). Such plutonium is produced from fuel rods that have been irradiated a very short time as measured in MW-Day/Ton burnup
Burnup
In nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source...
. Such low irradiation times limit the amount of additional neutron capture
Neutron capture
Neutron capture is a kind of nuclear reaction in which an atomic nucleus collides with one or more neutrons and they merge to form a heavier nucleus. Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled...
and therefore buildup of alternate isotope products such as Pu-240 in the rod, and also by consequence is considerably more expensive to produce, needing far more rods irradiated and processed for a given amount of plutonium.
Plutonium-240, in addition to being a neutron emitter after fission, is a gamma emitter in that process as well, and so is responsible for a large fraction of the radiation from stored nuclear weapons. Submarine
Submarine
A submarine is a watercraft capable of independent operation below the surface of the water. It differs from a submersible, which has more limited underwater capability...
crew members routinely operate in close proximity to stored weapons in torpedo rooms, unlike Air Force
Air force
An air force, also known in some countries as an air army, is in the broadest sense, the national military organization that primarily conducts aerial warfare. More specifically, it is the branch of a nation's armed services that is responsible for aerial warfare as distinct from an army, navy or...
missiles where exposures are relatively brief - hence justifying the additional costs of the premium supergrade alloy used on many naval nuclear torpedo weapons. Supergrade plutonium is used in W80 warheads.
Plutonium-239 in nuclear power reactors
In any operating nuclear reactor containing U-238, some plutonium-239 will accumulate in the nuclear fuel. Unlike reactors used to produce weapons-grade plutonium, commercial nuclear power reactors typically operate at a high burnupBurnup
In nuclear power technology, burnup is a measure of how much energy is extracted from a primary nuclear fuel source...
that allows a significant amount of plutonium to build up in irradiated reactor fuel. Plutonium-239 will be present both in the reactor core during operation and in spent nuclear fuel
Spent nuclear fuel
Spent nuclear fuel, occasionally called used nuclear fuel, is nuclear fuel that has been irradiated in a nuclear reactor...
that has been removed from the reactor at the end of the fuel assembly’s service life (typically several years). Spent nuclear fuel commonly contains about 0.8% plutonium-239.
Plutonium-239 present in reactor fuel can absorb neutrons and fission just as uranium-235 can. Since plutonium-239 is constantly being created in the reactor core during operation, the use of plutonium-239 as nuclear fuel in power plants can occur without reprocessing of spent fuel
Nuclear reprocessing
Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from irradiated nuclear fuel. Reprocessing serves multiple purposes, whose relative importance has changed over time. Originally reprocessing was used solely to extract plutonium for producing...
; the plutonium-239 is fissioned in the same fuel rods in which it is produced. Fissioning of plutonium-239 provides about one-third of the total energy produced in a typical commercial nuclear power plant. Reactor fuel would accumulate much more than 0.8% plutonium-239 during its service life if some plutonium-239 were not constantly being “burned off” by fissioning.
A small percentage of plutonium-239 can be deliberately added to fresh nuclear fuel. Such fuel is called MOX (mixed oxide) fuel
MOX fuel
Mixed oxide fuel, commonly referred to as MOX fuel, is nuclear fuel that contains more than one oxide of fissile material. MOX fuel contains plutonium blended with natural uranium, reprocessed uranium, or depleted uranium. MOX fuel is an alternative to the low-enriched uranium fuel used in the...
, as it contains a mixture of uranium oxide (UO2) and plutonium oxide (PuO2). The addition of plutonium-239 reduces or eliminates the need to enrich the uranium
Enriched uranium
Enriched uranium is a kind of uranium in which the percent composition of uranium-235 has been increased through the process of isotope separation. Natural uranium is 99.284% 238U isotope, with 235U only constituting about 0.711% of its weight...
in the fuel.

