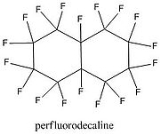
Perfluorodecalin
Encyclopedia
Perfluorodecalin is a fluorocarbon
, a derivative of decalin in which all of the hydrogen
atom
s are replaced by fluorine
atoms. It is chemically and biologically inert, and stable up to 400°C. Several applications make use of its ability to dissolve gases.
(the Fowler process
). The preferred starting material is tetralin
, having fewer hydrogens than decalin itself (and so requiring less fluorine), and a liquid, unlike naphthalene (so readily handled). For most applications, several steps of purification are required after reaction.
at STP
http://www.fluoros.co.uk/data/medical_applications/perfluorodecalin.php).
Perfluorodecalin was an ingredient in Fluosol
, an artificial blood product developed by Green Cross Corporation in the 1980s. It is also being studied for use in liquid breathing
. Perfluorodecalin can be applied topical
ly, to provide extra oxygen to a specific location, to accelerate wound healing. Organs and tissues can be stored for longer in oxygenated perfluorodecalin; the "two-layer method" uses perfluorodecalin and UW solution
to preserve tissue for pancreas transplants, for instance http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VJ0-4HKMRV1-1R&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=602ad95d31b613229c6156a114d3e11e.
Furthermore, it is sometimes used to dissolve teflon.
Fluorocarbon
Fluorocarbons, sometimes referred to as perfluorocarbons or PFCs, are organofluorine compounds that contain only carbon and fluorine bonded together in strong carbon–fluorine bonds. Fluoroalkanes that contain only single bonds are more chemically and thermally stable than alkanes...
, a derivative of decalin in which all of the hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s are replaced by fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
atoms. It is chemically and biologically inert, and stable up to 400°C. Several applications make use of its ability to dissolve gases.
Manufacture
Commercially, perfluorodecalin is manufactured by fluorination with cobalt(III) fluorideCobalt(III) fluoride
Cobalt fluoride is the inorganic compound with the formula CoF3. This highly reactive, hygroscopic brown solid is used to synthesize organofluorine compounds...
(the Fowler process
Fowler process
The Fowler Process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt fluoride.- Background :...
). The preferred starting material is tetralin
Tetralin
Tetralin is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.The compound can be synthesized in a Bergman cyclization...
, having fewer hydrogens than decalin itself (and so requiring less fluorine), and a liquid, unlike naphthalene (so readily handled). For most applications, several steps of purification are required after reaction.
Isomers
Perfluorodecalin exhibits cis-trans isomerism, as the tertiary fluorines atoms on the bridge carbon atoms can be either on the same side as each other (cis-isomer) or on opposite sides (trans-isomer). Both isomers are chemically and biologically inert, and are very similar in their physical properties. The most notable difference is in the melting point, which is -3.6°C for the cis-isomer, +18°C for the trans-isomer, and -6.7°C for a 50/50 mixture.Medical applications
Of all the perfluorocarbons, perfluorodecalin has probably seen the most interest in medical applications. Most applications utilize its ability to dissolve large amounts of oxygen (100 ml of perfluorodecalin at 25°C will dissolve 49 ml of oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
at STP
STP
- Commercial :* Straight Through Processing, banking term where a financial transaction is automatically completed without manual intervention* Segment, Target and Position, a marketing acronym...
http://www.fluoros.co.uk/data/medical_applications/perfluorodecalin.php).
Perfluorodecalin was an ingredient in Fluosol
Fluosol
Fluosol is an artificial blood substitute which is milky in color. Its main ingredients are perfluorodecalin or perfluorotributylamine in Fluosol-DA and Fluosol-43 respectively, perfluorochemicals suspended in an albumin emulsion. It was developed in Japan and first tested in the United States in...
, an artificial blood product developed by Green Cross Corporation in the 1980s. It is also being studied for use in liquid breathing
Liquid breathing
Liquid breathing is a form of respiration in which a normally air-breathing organism breathes an oxygen-rich liquid , rather than breathing air....
. Perfluorodecalin can be applied topical
Topical
In medicine, a topical medication is applied to body surfaces such as the skin or mucous membranes such as the vagina, anus, throat, eyes and ears.Many topical medications are epicutaneous, meaning that they are applied directly to the skin...
ly, to provide extra oxygen to a specific location, to accelerate wound healing. Organs and tissues can be stored for longer in oxygenated perfluorodecalin; the "two-layer method" uses perfluorodecalin and UW solution
Viaspan
Viaspan, also known as University of Wisconsin solution , was the first solution thoughtfully designed for use in organ transplantation, and became the first intracellular-like preservation medium...
to preserve tissue for pancreas transplants, for instance http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VJ0-4HKMRV1-1R&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000050221&_version=1&_urlVersion=0&_userid=10&md5=602ad95d31b613229c6156a114d3e11e.
Other applications
Due to its gas carrying capacity, perfluorodecalin has been used to enhance oxygen delivery during cell culture. Perfluorodecalin has also been shown to dramatically enhance in vivo microscopy resolution of airspace-containing tissues such as mesophyll http://www3.interscience.wiley.com/journal/123335070/abstract. Mounting leaves in perfluorodecalin significantly improves the optical qualities of the leaf, thereby enabling high-resolution imaging over twofold deeper into the mesophyll, compared with using water. The physiological impact of mounting the specimen in perfluorodecalin is also minimal compared to water http://www3.interscience.wiley.com/journal/123335070/abstract.Furthermore, it is sometimes used to dissolve teflon.

