
N-oxoammonium salt
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
are a class of organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s sharing a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
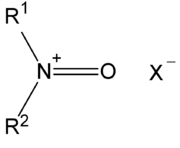
with the general structure R1R2N+=O X- where X- is the counterion
Counterion
A counterion is the ion that accompanies an ionic species in order to maintain electric neutrality. In table salt the sodium cation is the counterion for the chlorine anion and vice versa.In a charged transition metal complex, a simple A counterion is the ion that accompanies an ionic species in...
. The N-oxoammonium salt from TEMPO
TEMPO
oxyl, or oxidanyl or TEMPO is a chemical compound with the formula 32NO . This heterocycle is a red-orange, sublimable solid. As a stable radical, it has applications throughout chemistry and biochemistry. TEMPO was discovered by Lebedev and Kazarnowskii in 1960...
is used for oxidation of alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
groups.

