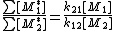Mayo-Lewis equation
Encyclopedia
The Mayo-Lewis equation or copolymer equation in polymer chemistry
describes the distribution of monomer
s in a copolymer :
Taking into consideration a monomer mix of two components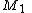 and
and 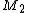 and the four different reactions that can take place at the reactive chain end terminating in either monomer (
and the four different reactions that can take place at the reactive chain end terminating in either monomer (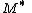 ) with their reaction rate constants
) with their reaction rate constants 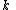 :
:




and with reactivity ratios defined as:
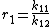
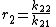
the copolymer equation is given as:
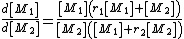
with the concentration
of the components given in square brackets. The equation gives the copolymer composition at any instant during the polymerization.
An example is maleic anhydride
and stilbene
, with reactivity ratio:
Both of these compounds do not homopolymerize and instead, they react together to give exclusively alternating copolymer.
Another form of the equation is:
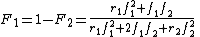
where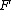 stands the mole fraction of each monomer in the copolymer:
stands the mole fraction of each monomer in the copolymer:
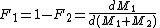
and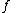 the mole fraction of each monomer in the feed:
the mole fraction of each monomer in the feed:
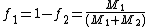
When the copolymer composition has the same composition as the feed, this composition is called the azeotrope.
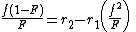
with
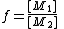 in the feed
in the feed
and
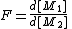 in the copolymer
in the copolymer
A number of copolymerization experiments are conducted with varying monomer ratios and the copolymer composition is analysed at low conversion. A plot of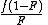 versus
versus 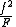 gives a straight line with slope
gives a straight line with slope 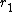 and intercept
and intercept 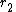 .
.
A semi-empirical method for the determination of reactivity ratios is called the Q-e scheme.
:
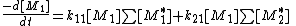
with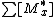 the concentration of all the active centers terminating in monomer 1 or 2.
the concentration of all the active centers terminating in monomer 1 or 2.
Likewise the rate of disappearance for monomer 2 is:
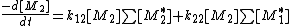
Division of both equations yields:
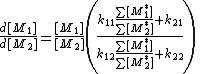
The ratio of active center concentrations can be found assuming steady state
with:
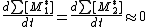
meaning that the concentration of active centres remains constant, the rate of formation for active center of monomer 1 is equal to the rate of their destruction or:
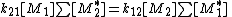
or
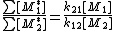
Polymer chemistry
Polymer chemistry or macromolecular chemistry is a multidisciplinary science that deals with the chemical synthesis and chemical properties of polymers or macromolecules. According to IUPAC recommendations, macromolecules refer to the individual molecular chains and are the domain of chemistry...
describes the distribution of monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s in a copolymer :
Taking into consideration a monomer mix of two components
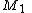 and
and 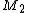 and the four different reactions that can take place at the reactive chain end terminating in either monomer (
and the four different reactions that can take place at the reactive chain end terminating in either monomer (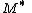 ) with their reaction rate constants
) with their reaction rate constants 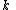 :
:



and with reactivity ratios defined as:
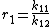
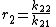
the copolymer equation is given as:
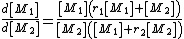
with the concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of the components given in square brackets. The equation gives the copolymer composition at any instant during the polymerization.
Limiting cases
From this equation several limiting cases can be derived:-
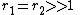 with both reactivity ratios very high the two monomers have no inclination to react to each other except with themselves leading to a mixture of two homopolymers.
with both reactivity ratios very high the two monomers have no inclination to react to each other except with themselves leading to a mixture of two homopolymers. -
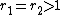 with both ratios larger than 1, homopolymerization of component M_1 is favored but in the event of a crosspolymerization by M_2 the chain-end will continue as such giving rise to block copolymer
with both ratios larger than 1, homopolymerization of component M_1 is favored but in the event of a crosspolymerization by M_2 the chain-end will continue as such giving rise to block copolymer -
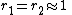 with both ratios around 1, monomer 1 will react as fast with another monomer 1 or monomer 2 and a random copolymer results.
with both ratios around 1, monomer 1 will react as fast with another monomer 1 or monomer 2 and a random copolymer results. -
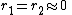 with both values approaching 0 the monomers are unable to react in homopolymerization and the result is an alternating polymer
with both values approaching 0 the monomers are unable to react in homopolymerization and the result is an alternating polymer -
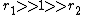 In the initial stage of the copolymerization monomer 1 is incorporated faster and the copolymer is rich in monomer 1. When this monomer gets depleted, more monomer 2 segments are added. This is called composition drift.
In the initial stage of the copolymerization monomer 1 is incorporated faster and the copolymer is rich in monomer 1. When this monomer gets depleted, more monomer 2 segments are added. This is called composition drift.
An example is maleic anhydride
Maleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
and stilbene
Stilbene
-Stilbene, is a diarylethene, i.e., a hydrocarbon consisting of a trans ethene double bond substituted with a phenyl group on both carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining....
, with reactivity ratio:
- Maleic anhydride (
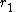 = 0.08) & cis-stilbene (
= 0.08) & cis-stilbene (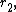 = 0.07)
= 0.07) - Maleic anhydride (
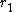 = 0.03) & trans-stilbene (
= 0.03) & trans-stilbene (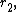 = 0.03)
= 0.03)
Both of these compounds do not homopolymerize and instead, they react together to give exclusively alternating copolymer.
Another form of the equation is:
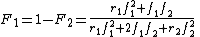
where
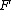 stands the mole fraction of each monomer in the copolymer:
stands the mole fraction of each monomer in the copolymer: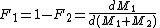
and
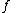 the mole fraction of each monomer in the feed:
the mole fraction of each monomer in the feed: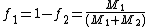
When the copolymer composition has the same composition as the feed, this composition is called the azeotrope.
Calculation of reactivity ratios
The reactivity ratios can be obtained by rewriting the copolymer equation to: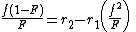
with
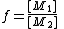 in the feed
in the feedand
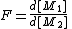 in the copolymer
in the copolymerA number of copolymerization experiments are conducted with varying monomer ratios and the copolymer composition is analysed at low conversion. A plot of
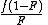 versus
versus 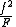 gives a straight line with slope
gives a straight line with slope 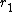 and intercept
and intercept 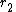 .
.A semi-empirical method for the determination of reactivity ratios is called the Q-e scheme.
Equation derivation
Monomer 1 is consumed with reaction rateReaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
:
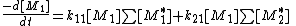
with
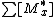 the concentration of all the active centers terminating in monomer 1 or 2.
the concentration of all the active centers terminating in monomer 1 or 2.Likewise the rate of disappearance for monomer 2 is:
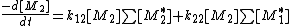
Division of both equations yields:
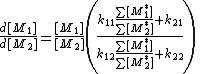
The ratio of active center concentrations can be found assuming steady state
Steady state (chemistry)
In chemistry, a steady state is a situation in which all state variables are constant in spite of ongoing processes that strive to change them. For an entire system to be at steady state, i.e. for all state variables of a system to be constant, there must be a flow through the system...
with:
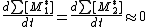
meaning that the concentration of active centres remains constant, the rate of formation for active center of monomer 1 is equal to the rate of their destruction or:
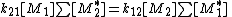
or