
Massieu function
Encyclopedia
In thermodynamics
, Massieu function, symbol (Psi), is defined by the following relation:
(Psi), is defined by the following relation:

where for every system
with degree of freedom r one may choose r variables, e.g. , to define a coordinate system, where X and Y are extensive and intensive
, to define a coordinate system, where X and Y are extensive and intensive
variables, respectively, and where at least one extensive variable must be within this set in order to define the size of the system. The (r + 1)-th variable,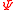 , is then called the Massieu function.
, is then called the Massieu function.
The Massieu function was introduced in the 1869 paper “On the Various Functions Characteristic of Fluids” by French engineer Francois Massieu
(1832-1896) and is sometimes called Massieu–Gibbs function, Massieu potential, or Gibbs function, or “characteristic function” in its original terminology. The name "Gibbs function" is the eponym of American physicist Willard Gibbs (1839-1903), who cited Massieu in his 1876 On the Equilibrium of Heterogeneous Substances
. Massieu, as discussed in the first footnote to the abstract of Gibbs' Equilibrium, “appears to have been the first to solve the problem of representing all the properties of a body of invariable composition which are concerned in reversible processes by means of a single function.” Massieu's 1869 paper seems to be the source for the generalized mathematical conception of the energy
of a system being equal to summations of the products of pairs of conjugate variables
.
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, Massieu function, symbol
 (Psi), is defined by the following relation:
(Psi), is defined by the following relation:
where for every system
System
System is a set of interacting or interdependent components forming an integrated whole....
with degree of freedom r one may choose r variables, e.g.
 , to define a coordinate system, where X and Y are extensive and intensive
, to define a coordinate system, where X and Y are extensive and intensiveIntensive
In grammar, an intensive word form is one which denotes stronger or more forceful action relative to the root on which the intensive is built. Intensives are usually lexical formations, but there may be a regular process for forming intensives from a root...
variables, respectively, and where at least one extensive variable must be within this set in order to define the size of the system. The (r + 1)-th variable,
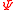 , is then called the Massieu function.
, is then called the Massieu function.The Massieu function was introduced in the 1869 paper “On the Various Functions Characteristic of Fluids” by French engineer Francois Massieu
Francois Massieu
In thermodynamics, Francois Jacques Dominique Massieu was a French engineer noted for his two 1869 characteristic functions, each of which known as a Massieu function , as cited by American engineer Willard Gibbs in his 1876 On the Equilibrium of Heterogeneous Substances.-External links:*Nivoit, E....
(1832-1896) and is sometimes called Massieu–Gibbs function, Massieu potential, or Gibbs function, or “characteristic function” in its original terminology. The name "Gibbs function" is the eponym of American physicist Willard Gibbs (1839-1903), who cited Massieu in his 1876 On the Equilibrium of Heterogeneous Substances
On the Equilibrium of Heterogeneous Substances
In the history of thermodynamics, On the Equilibrium of Heterogeneous Substances is a 300-page paper written by American mathematical-engineer Willard Gibbs...
. Massieu, as discussed in the first footnote to the abstract of Gibbs' Equilibrium, “appears to have been the first to solve the problem of representing all the properties of a body of invariable composition which are concerned in reversible processes by means of a single function.” Massieu's 1869 paper seems to be the source for the generalized mathematical conception of the energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
of a system being equal to summations of the products of pairs of conjugate variables
Conjugate variables (thermodynamics)
In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature/entropy or pressure/volume. In fact all thermodynamic potentials are expressed in terms of conjugate pairs....
.
Further reading
- Massieu, Francois. (1869). “Sur les Functions Caracteristiques des Divers Fluides et Sur la Theorie des Vapeurs”, Comptes Rendus, 69: 858–62, 1057–61.
- Massieu, Francois. (1876). Thermodynamique: Mêmoire sur les fonctions catactéristiques des divers fluides et sur la théorie des vapeurs. 92 pgs. Académie des Sciences de L'Institut National de France.

