
Lever rule
Encyclopedia
The lever rule is a tool used to determine weight percentages of each phase of a binary
equilibrium phase diagram
. It is used to determine the percent weight of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus
.
LS. This tie line is drawn horizontally at the composition's temperature from one phase to another (here the liquid to the solids). The percent weight of element B at the liquidus is given by wl and the percent weight of element B at the solidus is given by ws. The percent weight of solid and liquid can then be calculated using the following lever rule equations:
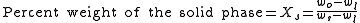
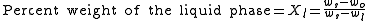
where wo is the percent weight of element B for the given composition.
The numerator of each equation is the original composition we are interested in +/- the Opposite lever arm. That is if you want the percent weight of solid then take the difference between the liquid composition and the original composition. And then the denominator is the overall length of the arm so the difference between the solid and liquid compositions.
Binary
- Mathematics :* Binary numeral system, a representation for numbers using only two digits * Binary function, a function in mathematics that takes two arguments- Computing :* Binary file, composed of something other than human-readable text...
equilibrium phase diagram
Phase diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases can occur at equilibrium...
. It is used to determine the percent weight of liquid and solid phases for a given binary composition and temperature that is between the liquidus and solidus
Solidus
Solidus may refer to:*Solidus , the "⁄" grammatical punctuation character, also used in mathematics*Slash a sign, "/" used as a punctuation mark and for various other purposes...
.
Binary Phase Diagrams
Before any calculations can be made a tie line is drawn on the phase diagram to determine the percent weight of each element; on the phase diagram to the right it is line segmentLine segment
In geometry, a line segment is a part of a line that is bounded by two end points, and contains every point on the line between its end points. Examples of line segments include the sides of a triangle or square. More generally, when the end points are both vertices of a polygon, the line segment...
LS. This tie line is drawn horizontally at the composition's temperature from one phase to another (here the liquid to the solids). The percent weight of element B at the liquidus is given by wl and the percent weight of element B at the solidus is given by ws. The percent weight of solid and liquid can then be calculated using the following lever rule equations:
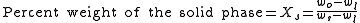
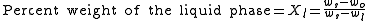
where wo is the percent weight of element B for the given composition.
The numerator of each equation is the original composition we are interested in +/- the Opposite lever arm. That is if you want the percent weight of solid then take the difference between the liquid composition and the original composition. And then the denominator is the overall length of the arm so the difference between the solid and liquid compositions.

