
L-selectride
Encyclopedia
L-selectride is an organoborane
. It is used in organic chemistry
as a reducing agent
, for example in the reduction of a ketone
, as part of Overman's synthesis of strychnine
.
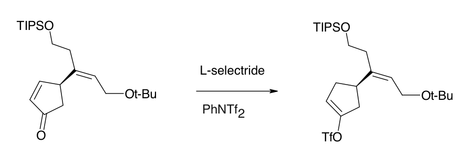
Under certain conditions, L-selectride can selectively reduce enone
s by conjugate addition of hydride, owing to the greater steric hindrance the bulky hydride reagent experiences at the carbonyl carbon relative to the (also-electrophilic) β-position. L-Selectride can also stereoselectively reduce carbonyl groups in a 1,2-fashion, again due to the steric nature of the hydride reagent.
N-selectride and K-selectride are related compounds, but instead of lithium as cation they have sodium
and potassium
cations respectively.
Organoborane
Organoborane or organoboron compounds are chemical compounds that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds...
. It is used in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
as a reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
, for example in the reduction of a ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
, as part of Overman's synthesis of strychnine
Strychnine
Strychnine is a highly toxic , colorless crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine causes muscular convulsions and eventually death through asphyxia or sheer exhaustion...
.

Under certain conditions, L-selectride can selectively reduce enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
s by conjugate addition of hydride, owing to the greater steric hindrance the bulky hydride reagent experiences at the carbonyl carbon relative to the (also-electrophilic) β-position. L-Selectride can also stereoselectively reduce carbonyl groups in a 1,2-fashion, again due to the steric nature of the hydride reagent.
N-selectride and K-selectride are related compounds, but instead of lithium as cation they have sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
cations respectively.

