
ITIES
Encyclopedia
ITIES is an acronym used in electrochemistry for the "Interface
between Two Immiscible Electrolyte
Solutions". Usually, one electrolyte is an aqueous electrolyte composed of hydrophilic ions such as NaCl dissolved in water and the other electrolyte is a lipophilic salt such as tetrabutylammonium tetraphenylborate
dissolved in an organic solvent immiscible with water such as nitrobenzene
, or 1,2-dichloroethane
.
An ITIES is an electrochemical interface that is either polarisable or polarised. An ITIES is said polarisable if one can change the Galvani potential difference or in other words the difference of inner potentials between the two adjacent phases without changing noticeably the chemical composition of the respective phases, i.e. without noticeable electrochemical reactions taking place at the interface. An ITIES system is said polarised if the distribution of the different charges and redox species between the two phases determines the Galvani potential
difference.
 The Nernst equation
The Nernst equation
for an ion transfer reaction reads
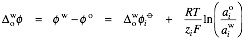 ,
,
where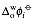 is the standard transfer potential defined as the Gibbs energy of transfer expressed in a voltage scale.
is the standard transfer potential defined as the Gibbs energy of transfer expressed in a voltage scale.
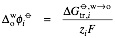
The Nernst equation for a single heterogeneous electron transfer reaction reads
,
where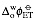 is the standard redox potential for the interfacial transfer of electrons defined as the difference the standard redox potentials of the two redox couples but referred to the aqueous Standard Hydrogen Electrode (SHE).
is the standard redox potential for the interfacial transfer of electrons defined as the difference the standard redox potentials of the two redox couples but referred to the aqueous Standard Hydrogen Electrode (SHE).
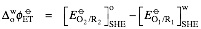
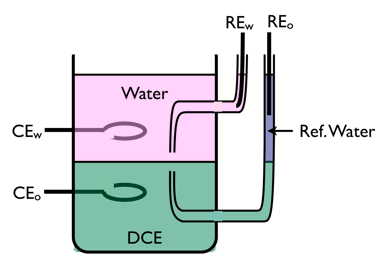
Two reference electrodes are used to control the polarisation of the interface, and two counter electrodes made of noble metals are used to pass the current. The aqueous supporting electrolyte must be hydrophilic, e.g. LiCl, and the organic electrolyte must be lipophilic, e.g. tetraheptylammonium tetra-pentafluorophenyl borate.
where γ represents the activity coefficient.
Interface (chemistry)
An interface is a surface forming a common boundary among two different phases, such as an insoluble solid and a liquid, two immiscible liquids or a liquid and an insoluble gas. The importance of the interface depends on which type of system is being treated: the bigger the quotient area/volume,...
between Two Immiscible Electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
Solutions". Usually, one electrolyte is an aqueous electrolyte composed of hydrophilic ions such as NaCl dissolved in water and the other electrolyte is a lipophilic salt such as tetrabutylammonium tetraphenylborate
Tetraphenylborate
Tetraphenylborate is an organoboron anion consisting of a central boron atom with four phenyl groups. Tetraphenylborate uncouples oxidative phosphorylation....
dissolved in an organic solvent immiscible with water such as nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
, or 1,2-dichloroethane
1,2-Dichloroethane
The chemical compound 1,2-dichloroethane, commonly known by its old name of ethylene dichloride , is a chlorinated hydrocarbon, mainly used to produce vinyl chloride monomer , the major precursor for PVC production. It is a colourless liquid with a chloroform-like odour...
.
An ITIES is an electrochemical interface that is either polarisable or polarised. An ITIES is said polarisable if one can change the Galvani potential difference or in other words the difference of inner potentials between the two adjacent phases without changing noticeably the chemical composition of the respective phases, i.e. without noticeable electrochemical reactions taking place at the interface. An ITIES system is said polarised if the distribution of the different charges and redox species between the two phases determines the Galvani potential
Galvani potential
Galvani potential in electrochemistry, is the electric potential difference between two points in the bulk of two phases...
difference.
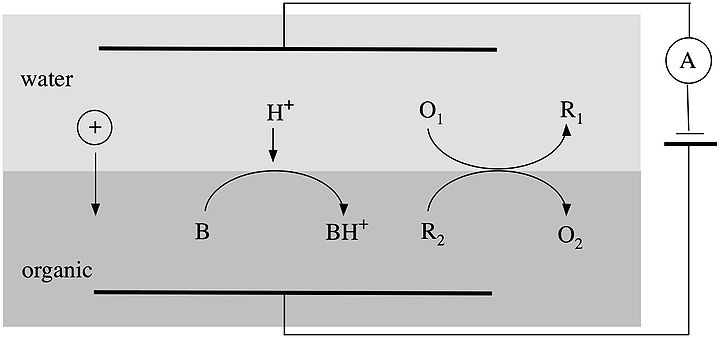
Charge transfer reactions at ITIES
Three major classes of charge transfer reactions can be studied at ITIES:- Ion transfer reactions.
- Assisted ion transfer reactions.
- Heterogeneous electron transfer reactions.

Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
for an ion transfer reaction reads
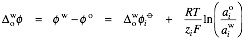 ,
,where
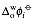 is the standard transfer potential defined as the Gibbs energy of transfer expressed in a voltage scale.
is the standard transfer potential defined as the Gibbs energy of transfer expressed in a voltage scale. 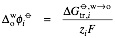
The Nernst equation for a single heterogeneous electron transfer reaction reads
,
where
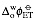 is the standard redox potential for the interfacial transfer of electrons defined as the difference the standard redox potentials of the two redox couples but referred to the aqueous Standard Hydrogen Electrode (SHE).
is the standard redox potential for the interfacial transfer of electrons defined as the difference the standard redox potentials of the two redox couples but referred to the aqueous Standard Hydrogen Electrode (SHE).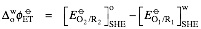
Four-Electrode cell
To study charge transfer reactions at ITIES, a 4-electrode cell is used.
Two reference electrodes are used to control the polarisation of the interface, and two counter electrodes made of noble metals are used to pass the current. The aqueous supporting electrolyte must be hydrophilic, e.g. LiCl, and the organic electrolyte must be lipophilic, e.g. tetraheptylammonium tetra-pentafluorophenyl borate.
Ion partition coefficient/Ion distribution coefficient
Contrary to a neutral solute, the partition coefficient of an ion depends of the Galvani potential difference between the two phasesDistribution potential
When a salt is distributed between two phases, the Galvani potential difference is called the distribution potential and is obtained from the respective Nernst equations for the cation C+ and the anion A– to readwhere γ represents the activity coefficient.

