
Frustrated Lewis pair
Encyclopedia
In chemistry, a frustrated Lewis pair is a compound or mixture containing a Lewis acid
and a Lewis base that, because of steric hindrance, cannot combine to form an adduct
. Because of their "unquenched" reactivity, such systems are very reactive and are able to split the hydrogen molecule heterolytically, which makes them potentially useful as metal-free catalysts for hydrogenation
reactions.
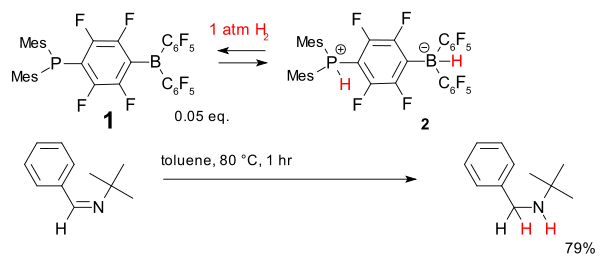
In the scheme below, compound 1 has a frustrated Lewis pair: the lone pair on the phosphorus atoms cannot be donated to the boron atoms because of the large substituents on both atoms. However, when exposed to hydrogen at 1 atm, the zwitterion
ic compound 2 is formed. This hydrogen addition is reversible, and it is possible to transfer the activated hydrogen to a sterically hindered imine, which results in a catalytic cycle that produces the hydrogenated product, using only 0.05 equivalents of 1 as a catalyst.
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
and a Lewis base that, because of steric hindrance, cannot combine to form an adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
. Because of their "unquenched" reactivity, such systems are very reactive and are able to split the hydrogen molecule heterolytically, which makes them potentially useful as metal-free catalysts for hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
reactions.
In the scheme below, compound 1 has a frustrated Lewis pair: the lone pair on the phosphorus atoms cannot be donated to the boron atoms because of the large substituents on both atoms. However, when exposed to hydrogen at 1 atm, the zwitterion
Zwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
ic compound 2 is formed. This hydrogen addition is reversible, and it is possible to transfer the activated hydrogen to a sterically hindered imine, which results in a catalytic cycle that produces the hydrogenated product, using only 0.05 equivalents of 1 as a catalyst.


