
Ethylbenzene hydroxylase
Encyclopedia
In enzymology, an ethylbenzene hydroxylase is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are ethylbenzene
, H2O
, and acceptor
, whereas its two products
are (S)-1-phenylethanol and reduced acceptor.
This enzyme belongs to the family of oxidoreductase
s, specifically those acting on CH or CH2 group with other acceptors. The systematic name of this enzyme class is ethylbenzene:acceptor oxidoreductase. Other names in common use include ethylbenzene dehydrogenase, and ethylbenzene:(acceptor) oxidoreductase. This enzyme participates in ethylbenzene degradation by Aromatoleum aromaticum, a denitrifying bacterium related to the genera Azoarcus and Thauera. It is a molybdenum enzyme belonging to the DMSO reductase
family. Molybdenum enzymes are distinguished by the presence of a unique active site containing molybdenum atom, one or two molybdopterins
and additional ligands (i.e. aminoacid residue of Ser, Cys, SeCys or Asp and very often oxygen Mo=O ligand). EBDH is synthesized exclusively in cells grown anaerobically on ethylbenzene and has been identified as a soluble periplasmic protein
has been solved for this class of enzymes, with the PDB
accession code . EBDH consists of three subunits of 96, 43, and 23 kDa, and contains a molybdenum cofactor and a heme b559 cofactor linked by a linear row of five iron-sulfur clusters.
The EBDH catalytic cycle has two parts: i) oxidation part, where substrate is oxidized to alcohols and the enzyme is reduced to its catalytically inactive form, and ii) enzyme re-oxidation part, where EBDH active site (MoCo) is oxidized and restored to its catalytically active form.
Recent theoretical and experimental studies point toward radical C-H activation as the initial reaction and rate limiting step. A possible alternative hydride transfer seems to be less likely. The mechanism concludes with conversion of the hydrocarbon to a carbocation intermediate and rebound of a hydroxide to form the hydroxylated product. Moreover, a histidine residue (His192) of the active site seems to be involved in the reaction mechanism.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- ethylbenzene + H2O + acceptor
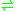 (S)-1-phenylethanol + reduced acceptor
(S)-1-phenylethanol + reduced acceptor
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are ethylbenzene
Ethylbenzene
Ethylbenzene is an organic compound with the formula C6H5CH2CH3. This aromatic hydrocarbon is important in the petrochemical industry as an intermediate in the production of styrene, which in turn is used for making polystyrene, a common plastic material....
, H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, and acceptor
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process....
, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are (S)-1-phenylethanol and reduced acceptor.
This enzyme belongs to the family of oxidoreductase
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s, specifically those acting on CH or CH2 group with other acceptors. The systematic name of this enzyme class is ethylbenzene:acceptor oxidoreductase. Other names in common use include ethylbenzene dehydrogenase, and ethylbenzene:(acceptor) oxidoreductase. This enzyme participates in ethylbenzene degradation by Aromatoleum aromaticum, a denitrifying bacterium related to the genera Azoarcus and Thauera. It is a molybdenum enzyme belonging to the DMSO reductase
DMSO reductase
DMSO reductase is a molybdenum-containing enzyme capable of reducing dimethyl sulfoxide to dimethyl sulfide . This enzyme serves as the terminal reductase under anaerobic conditions in some bacteria, with DMSO being the terminal electron acceptor...
family. Molybdenum enzymes are distinguished by the presence of a unique active site containing molybdenum atom, one or two molybdopterins
Molybdopterin
Molybdopterins, when reacted with molybdenum or tungsten in the form of molybdate or tungstate, are a class of cofactors found in most molybdenum and all tungsten enzymes...
and additional ligands (i.e. aminoacid residue of Ser, Cys, SeCys or Asp and very often oxygen Mo=O ligand). EBDH is synthesized exclusively in cells grown anaerobically on ethylbenzene and has been identified as a soluble periplasmic protein
Structural studies
As of late 2007, only one structureTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
has been solved for this class of enzymes, with the PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession code . EBDH consists of three subunits of 96, 43, and 23 kDa, and contains a molybdenum cofactor and a heme b559 cofactor linked by a linear row of five iron-sulfur clusters.
Mechanism
The reaction is catalyzed by the enzyme using a molybdenum cofactor (MoCo), which in the native state consists of a molybdenum (VI) nucleus ligated by two molybdopterin guanine dinucleotide (MGD) ligands and an aspartic acid residue. Two electrons acquired by the cofactor during the reaction, i.e., the hydroxylation of the hydrocarbon, are then transferred via a chain of iron-sulfur clusters connecting the molybdenum with a heme b cofactor in the alpha subunit, from which the electrons are donated to a yet-unknown acceptor. Notably, EBDH exhibits in vitro activity only with artificial electron acceptors of high redox potential, like the ferricenium ion (E0’= +380 mV). This suggests that its natural electron acceptor may be a periplasmic cytochrome c of similarly high potential, which would couple the ethylbenzene oxidation to the nitrate respiration of A. aromaticum.The EBDH catalytic cycle has two parts: i) oxidation part, where substrate is oxidized to alcohols and the enzyme is reduced to its catalytically inactive form, and ii) enzyme re-oxidation part, where EBDH active site (MoCo) is oxidized and restored to its catalytically active form.
Recent theoretical and experimental studies point toward radical C-H activation as the initial reaction and rate limiting step. A possible alternative hydride transfer seems to be less likely. The mechanism concludes with conversion of the hydrocarbon to a carbocation intermediate and rebound of a hydroxide to form the hydroxylated product. Moreover, a histidine residue (His192) of the active site seems to be involved in the reaction mechanism.

