Ethanol metabolism
Encyclopedia
Ethanol
is metabolized through a very complex catabolic metabolic pathway
.
s and organs allows for greater specificity of function. This occurs for the processing of ethanol in the human body. We find that all the enzymes needed to accomplish the oxidation reactions are confined to certain tissues. In particular, we find much higher concentration of such enzymes in the kidneys and in the liver
, making such organs the primary site for alcohol catabolism.
and water
is a complex one that proceeds in three steps. Below, the Gibbs Free Energy
of Formation for each step is shown with ΔGf values given in the CRC.
Complete Reaction:
C2H6O(Ethanol)→C2H4O(Acetaldehyde)→C2H4O2(acetic Acid) →Acetyl-CoA→3H2O+2CO2.
ΔGf = Σ ΔGfp - ΔGfo
Step One:
Ethanol: -174.8 kJ/mol
Ethanal(Acetaldehyde): -127.6 kJ/mol
ΔGf1 = -127.6 + 174.8 = 47.2 kJ/mol(Endergonic)
ΣΔGf = 47.2 kJ/mol (Endergonic)
Step Two:
Ethanal: -127.6 kJ/mol
Acetic Acid: -389.9 kJ/mol
ΔGf2 = -389.9 + 127.6 = -262.3 kJ/mol (Exergonic)
ΣΔGf = -215.1 kJ/mol (Exergonic)
Step Four: (Because the Gibbs energy is a state function, we can skip the Acetyl-CoA (step 3), for which themodynamic values are not known).
Acetic Acid: -389.9 kJ/mol
3H2O+2CO2: -1500.1 kJ/mol
ΔGf4 = -1500 + 389.6 = -1110.5 kJ/mol (Exergonic)
ΣΔGf = -1325.3 kJ/mol (Exergonic)
) per molecule of ethanol.
to acetic acid
to acetyl-CoA
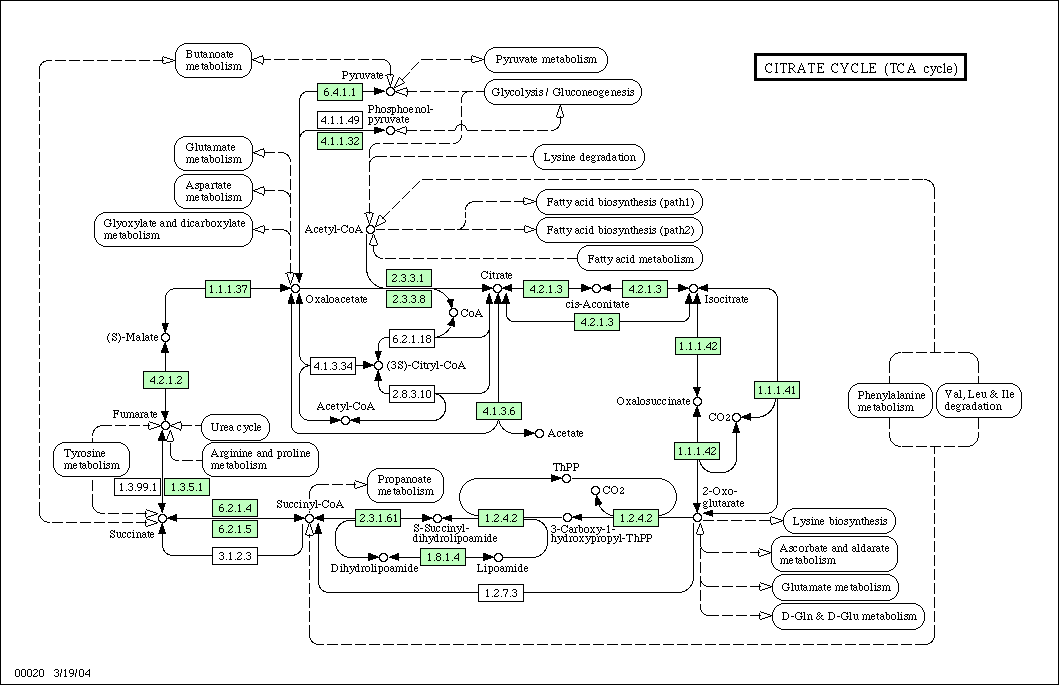
. Once acetyl-CoA is formed, it is free to enter directly into the citric acid cycle
.
via the enzyme alcohol dehydrogenase IB (class I), beta polypeptide (ADH1B). The gene coding for this enzyme is 1.1.1.1 on chromosome 4, locus 4q21-q23. The enzyme "encoded by this gene is a member of the alcohol dehydrogenase family. Members of this enzyme family metabolize a wide variety of substrates, including ethanol, retinol, other aliphatic alcohols, hydroxysteroids, and lipid peroxidation products. This encoded protein, consisting of several homo- and heterodimers of alpha, beta, and gamma subunits, exhibits high activity for ethanol oxidation and plays a major role in ethanol catabolism. Three genes encoding alpha, beta and gamma subunits are tandemly organized in a genomic segment as a gene cluster."
(Vitamin C
) and Vitamin B1 (thiamine
). These free radicals can result in damage to embryonic neural crest cells and can lead to severe birth defects. Prolonged exposure of the kidney and liver to these compounds in chronic alcoholics can lead to severe damage. The literature also suggests that these toxins may have a hand in causing some of the ill effects associated with hang-overs.
The enzyme associated with the chemical transformation from acetaldehyde to acetic acid is aldehyde dehydrogenase 2 family (ALDH2
). The gene encoding for this enzyme is 1.2.1.3 and is found on chromosome 12, locus q24.2.
"This enzyme is alcohol dehydrogenase 1A (class I), alpha polypeptide. This protein belongs to the aldehyde dehydrogenase family of proteins. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Two major liver isoforms of this enzyme, cytosolic and mitochondrial, can be distinguished by their electrophoretic mobilities, kinetic properties, and subcellular localizations. Most Caucasians have two major isozymes, while approximately 50% of Asians have only the cytosolic isozyme, missing the mitochondrial isozyme. A remarkably higher frequency of acute alcohol intoxication among Asians than among Caucasians could be related to the absence of the mitochondrial isozyme. This gene encodes a mitochondrial isoform, which has a low Km for ethanol, and is localized in mitochondrial matrix."
is ACSS2
; it is expressed by gene 6.2.1.1 located on
chromosome 20 locus q11.22. "This gene encodes a cytosolic enzyme that catalyzes the activation of acetate for use in lipid synthesis and energy generation. The protein acts as a monomer and produces acetyl-CoA from acetate in a reaction that requires ATP. Expression of this gene is regulated by sterol regulatory element-binding proteins, transcription factors that activate genes required for the synthesis of cholesterol and unsaturated fatty acids. Two transcript variants encoding different isoforms have been found for this gene."
Gene 6.2.1.1 on Chromosome 20
.
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
is metabolized through a very complex catabolic metabolic pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
.
Ethanol and evolution
The average human digestive system produces approximately 3g of ethanol per day merely through fermentation of its contents. Catabolic degradation of ethanol is thus essential to life, not only of humans, but of almost all living organisms. In fact, certain amino acid sequences in the enzymes used to oxidize ethanol are conserved all the way back to single cell bacteria. Such a functionality is needed because all organisms actually produce alcohol in small amounts by several pathways, primary amongst the fatty acid synthesis, glycerolipid metabolism, and bile acid biosynthesis. If the body had no mechanism for catabolizing the alcohols, they would build up in the body and become toxic. This could be an evolutionary rationale for alcohol catabolism also by sulfotransferase.Physiologic structures
As is a basic organizing theme in biological systems, greater complexity of a body system, such as tissueBiological tissue
Tissue is a cellular organizational level intermediate between cells and a complete organism. A tissue is an ensemble of cells, not necessarily identical, but from the same origin, that together carry out a specific function. These are called tissues because of their identical functioning...
s and organs allows for greater specificity of function. This occurs for the processing of ethanol in the human body. We find that all the enzymes needed to accomplish the oxidation reactions are confined to certain tissues. In particular, we find much higher concentration of such enzymes in the kidneys and in the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
, making such organs the primary site for alcohol catabolism.
Energy Calculations
The reaction from ethanol to carbon dioxideCarbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
is a complex one that proceeds in three steps. Below, the Gibbs Free Energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of Formation for each step is shown with ΔGf values given in the CRC.
Complete Reaction:
C2H6O(Ethanol)→C2H4O(Acetaldehyde)→C2H4O2(acetic Acid) →Acetyl-CoA→3H2O+2CO2.
ΔGf = Σ ΔGfp - ΔGfo
Step One:
Ethanol: -174.8 kJ/mol
Ethanal(Acetaldehyde): -127.6 kJ/mol
ΔGf1 = -127.6 + 174.8 = 47.2 kJ/mol(Endergonic)
ΣΔGf = 47.2 kJ/mol (Endergonic)
Step Two:
Ethanal: -127.6 kJ/mol
Acetic Acid: -389.9 kJ/mol
ΔGf2 = -389.9 + 127.6 = -262.3 kJ/mol (Exergonic)
ΣΔGf = -215.1 kJ/mol (Exergonic)
Step Four: (Because the Gibbs energy is a state function, we can skip the Acetyl-CoA (step 3), for which themodynamic values are not known).
Acetic Acid: -389.9 kJ/mol
3H2O+2CO2: -1500.1 kJ/mol
ΔGf4 = -1500 + 389.6 = -1110.5 kJ/mol (Exergonic)
ΣΔGf = -1325.3 kJ/mol (Exergonic)
Discussion of calculations
If catabolism of alcohol goes all the way to completion, then, we have a very exothermic event yielding some 1325 kJ/mol of energy. If the reaction stops part way through the metabolic pathways, which happens because acetic acid is excreted in the urine after drinking, then not nearly as much energy can be derived from alcohol, indeed, only 215.1 kJ/mol. At the very least, the theoretical limits on energy yield are determined to be 215.1 kJ/mol to 1325.3 kJ/mol. It is also important to note that step 1 on this reaction is endothermic, requiring 47.2 kJ/mol of alcohol, or about 3 molecules of ATP (adenosine triphosphateAdenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
) per molecule of ethanol.
Steps of the reaction
The first three steps of the reaction pathways lead from ethanol to acetaldehydeAcetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
to acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
to acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
. Once acetyl-CoA is formed, it is free to enter directly into the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
.
Organic reactions
The reactions that transform ethanol into an aldehyde and then into a carboxylic acid are examples of oxidation reactions, which in organic chemistry, are typically characterized by the addition of oxygen onto a functional group. The third reaction, the enzyme mediated formation of acetyl-CoA from acetic acid is an example of an enzymatic synthetase reaction where, through a complex intramolecular interaction a product molecule is formed from reactants.Ethanol to acetaldehyde
Ethanol is oxidized to acetaldehydeAcetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
via the enzyme alcohol dehydrogenase IB (class I), beta polypeptide (ADH1B). The gene coding for this enzyme is 1.1.1.1 on chromosome 4, locus 4q21-q23. The enzyme "encoded by this gene is a member of the alcohol dehydrogenase family. Members of this enzyme family metabolize a wide variety of substrates, including ethanol, retinol, other aliphatic alcohols, hydroxysteroids, and lipid peroxidation products. This encoded protein, consisting of several homo- and heterodimers of alpha, beta, and gamma subunits, exhibits high activity for ethanol oxidation and plays a major role in ethanol catabolism. Three genes encoding alpha, beta and gamma subunits are tandemly organized in a genomic segment as a gene cluster."
Acetaldehyde to acetic acid
Acetaldehyde is a highly unstable compound and quickly forms free radical structures which are highly toxic if not quenched by antioxidants such as ascorbic acidAscorbic acid
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. It is a white solid, but impure samples can appear yellowish. It dissolves well in water to give mildly acidic solutions. Ascorbic acid is one form of vitamin C. The name is derived from a- and scorbutus , the...
(Vitamin C
Vitamin C
Vitamin C or L-ascorbic acid or L-ascorbate is an essential nutrient for humans and certain other animal species. In living organisms ascorbate acts as an antioxidant by protecting the body against oxidative stress...
) and Vitamin B1 (thiamine
Thiamine
Thiamine or thiamin or vitamin B1 , named as the "thio-vitamine" is a water-soluble vitamin of the B complex. First named aneurin for the detrimental neurological effects if not present in the diet, it was eventually assigned the generic descriptor name vitamin B1. Its phosphate derivatives are...
). These free radicals can result in damage to embryonic neural crest cells and can lead to severe birth defects. Prolonged exposure of the kidney and liver to these compounds in chronic alcoholics can lead to severe damage. The literature also suggests that these toxins may have a hand in causing some of the ill effects associated with hang-overs.
The enzyme associated with the chemical transformation from acetaldehyde to acetic acid is aldehyde dehydrogenase 2 family (ALDH2
ALDH2
Aldehyde dehydrogenase 2 family , also known as ALDH2, is a human gene found on chromosome 12.-Function:The enzyme encoded by this gene belongs to the aldehyde dehydrogenase family of enzymes that catalyze the chemical transformation from acetaldehyde to acetic acid...
). The gene encoding for this enzyme is 1.2.1.3 and is found on chromosome 12, locus q24.2.
"This enzyme is alcohol dehydrogenase 1A (class I), alpha polypeptide. This protein belongs to the aldehyde dehydrogenase family of proteins. Aldehyde dehydrogenase is the second enzyme of the major oxidative pathway of alcohol metabolism. Two major liver isoforms of this enzyme, cytosolic and mitochondrial, can be distinguished by their electrophoretic mobilities, kinetic properties, and subcellular localizations. Most Caucasians have two major isozymes, while approximately 50% of Asians have only the cytosolic isozyme, missing the mitochondrial isozyme. A remarkably higher frequency of acute alcohol intoxication among Asians than among Caucasians could be related to the absence of the mitochondrial isozyme. This gene encodes a mitochondrial isoform, which has a low Km for ethanol, and is localized in mitochondrial matrix."
Acetic acid to acetyl-CoA
The enzyme associated with the conversion of acetic acid to acetyl-CoAAcetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
is ACSS2
ACSS2
Acetyl-coenzyme A synthetase, cytoplasmic is an enzyme that in humans is encoded by the ACSS2 gene.-Further reading:...
; it is expressed by gene 6.2.1.1 located on
chromosome 20 locus q11.22. "This gene encodes a cytosolic enzyme that catalyzes the activation of acetate for use in lipid synthesis and energy generation. The protein acts as a monomer and produces acetyl-CoA from acetate in a reaction that requires ATP. Expression of this gene is regulated by sterol regulatory element-binding proteins, transcription factors that activate genes required for the synthesis of cholesterol and unsaturated fatty acids. Two transcript variants encoding different isoforms have been found for this gene."
Gene 6.2.1.1 on Chromosome 20
Acetyl-CoA to water and carbon dioxide
Once acetyl-CoA is formed it enters the normal citric acid cycleCitric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
.


