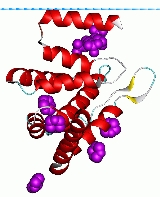
ENTH domain
Encyclopedia
The Epsin N-terminal homology (ENTH) domain is a structural domain that is found in proteins involved in endocytosis
and cytoskeletal machinery.
varying length. The general topology is determined by three helical hairpins that are stacked consecutively with a right hand twist. An N-terminal helix folds back, forming a deep basic groove that forms the binding pocket for the Ins(1,4,5)P3 ligand. The lipid ligand is coordinated by residues from surrounding alpha-helices and all three phosphates are multiply coordinated.
suggesting that the domain is a membrane interacting module. The main function of proteins containing this domain appears to be to act as accessory clathrin
adaptors in endocytosis, Epsin is able to recruit and promote clathrin polymerisation on a lipid monolayer, but
may have additional roles in signalling and actin regulation. Epsin causes a strong degree of membrane curvature and tubulation, even fragmentation of membranes with a high PtdIns(4,5)P2 content. Epsin binding to membranes facilitates their deformation by insertion of the N-terminal helix into the outer leaflet of
the bilayer, pushing the head groups apart. This would reduce the energy needed to curve the membrane into a vesicle, making it easier for the clathrin cage to fix and stabilise the curved membrane. This points to a pioneering role for epsin in vesicle budding as it provides both a driving force and a link between membrane invagination and clathrin polymerisation.
In particular, Epsin-1 shows specificity for the membrane glycophospholipid phosphatidylinositol-4,5-bisphosphate, however not all ENTH domains bind to this molecule. Binding causes tubulation of liposome
s and in vivo this membrane-binding function is normally coordinated with clathrin
polymerisation.
The N-terminal alpha-helix of this domain is hydrophobic and inserts into the membrane like a wedge and helps to drive membrane curvature.
Endocytosis
Endocytosis is a process by which cells absorb molecules by engulfing them. It is used by all cells of the body because most substances important to them are large polar molecules that cannot pass through the hydrophobic plasma or cell membrane...
and cytoskeletal machinery.
Structure
This domain is approximately 150 amino acids in length and is always found located at the N-termini of proteins. The domain forms a compact globular structure, composed of 9 alpha-helices connected by loops ofvarying length. The general topology is determined by three helical hairpins that are stacked consecutively with a right hand twist. An N-terminal helix folds back, forming a deep basic groove that forms the binding pocket for the Ins(1,4,5)P3 ligand. The lipid ligand is coordinated by residues from surrounding alpha-helices and all three phosphates are multiply coordinated.
Interactions with the lipid bilayer
Proteins containing this domain have been found to bind PtdIns(4,5)P2 and PtdIns(1,4,5)P3Inositol triphosphate
Inositol trisphosphate or inositol 1,4,5-trisphosphate , together with diacylglycerol , is a secondary messenger molecule used in signal transduction and lipid signaling in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell...
suggesting that the domain is a membrane interacting module. The main function of proteins containing this domain appears to be to act as accessory clathrin
Clathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1975. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice...
adaptors in endocytosis, Epsin is able to recruit and promote clathrin polymerisation on a lipid monolayer, but
may have additional roles in signalling and actin regulation. Epsin causes a strong degree of membrane curvature and tubulation, even fragmentation of membranes with a high PtdIns(4,5)P2 content. Epsin binding to membranes facilitates their deformation by insertion of the N-terminal helix into the outer leaflet of
the bilayer, pushing the head groups apart. This would reduce the energy needed to curve the membrane into a vesicle, making it easier for the clathrin cage to fix and stabilise the curved membrane. This points to a pioneering role for epsin in vesicle budding as it provides both a driving force and a link between membrane invagination and clathrin polymerisation.
In particular, Epsin-1 shows specificity for the membrane glycophospholipid phosphatidylinositol-4,5-bisphosphate, however not all ENTH domains bind to this molecule. Binding causes tubulation of liposome
Liposome
Liposomes are artificially prepared vesicles made of lipid bilayer. Liposomes can be filled with drugs, and used to deliver drugs for cancer and other diseases. Liposomes are composite structures made of phospholipids and may contain small amounts of other molecules...
s and in vivo this membrane-binding function is normally coordinated with clathrin
Clathrin
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1975. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice...
polymerisation.
The N-terminal alpha-helix of this domain is hydrophobic and inserts into the membrane like a wedge and helps to drive membrane curvature.
External links
- Endocytosis.org entry on epsin
- ENTH domain in PROSITEPROSITEPROSITE is a protein database. It consists of entries describing the protein families, domains and functional sites as well as amino acid patterns, signatures, and profiles in them. These are manually curated by a team of the Swiss Institute of Bioinformatics and tightly integrated into Swiss-Prot...

