
Deoxycytidylate 5-hydroxymethyltransferase
Encyclopedia
In enzymology, a deoxycytidylate 5-hydroxymethyltransferase is an enzyme
that catalyzes
the chemical reaction
The 3 substrates
of this enzyme are 5,10-methylenetetrahydrofolate
, H2O
, and deoxycytidylate, whereas its two products
are tetrahydrofolate and 5-hydroxymethyldeoxycytidylate.
This enzyme belongs to the family of transferase
s that transfer one-carbon groups, specifically the hydroxymethyl-, formyl- and related transferases. The systematic name of this enzyme class is 5,10-methylenetetrahydrofolate:deoxycytidylate 5-hydroxymethyltransferase. Other names in common use include dCMP hydroxymethylase, d-cytidine 5'-monophosphate hydroxymethylase, deoxyCMP hydroxymethylase, deoxycytidylate hydroxymethylase, and deoxycytidylic hydroxymethylase. This enzyme participates in pyrimidine metabolism
and one carbon pool by folate.
have been solved for this class of enzymes, with PDB
accession codes , , and .
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
- 5,10-methylenetetrahydrofolate + H2O + deoxycytidylate
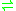 tetrahydrofolate + 5-hydroxymethyldeoxycytidylate
tetrahydrofolate + 5-hydroxymethyldeoxycytidylate
The 3 substrates
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of this enzyme are 5,10-methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate
5,10-Methylenetetrahydrofolate is the substrate used by the enzyme methylenetetrahydrofolate reductase to generate 5-methyltetrahydrofolate ....
, H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, and deoxycytidylate, whereas its two products
Product (chemistry)
Product are formed during chemical reactions as reagents are consumed. Products have lower energy than the reagents and are produced during the reaction according to the second law of thermodynamics. The released energy comes from changes in chemical bonds between atoms in reagent molecules and...
are tetrahydrofolate and 5-hydroxymethyldeoxycytidylate.
This enzyme belongs to the family of transferase
Transferase
In biochemistry, a transferase is an enzyme that catalyzes the transfer of a functional group from one molecule to another . For example, an enzyme that catalyzed this reaction would be a transferase:In this example, A would be the donor, and B would be the acceptor...
s that transfer one-carbon groups, specifically the hydroxymethyl-, formyl- and related transferases. The systematic name of this enzyme class is 5,10-methylenetetrahydrofolate:deoxycytidylate 5-hydroxymethyltransferase. Other names in common use include dCMP hydroxymethylase, d-cytidine 5'-monophosphate hydroxymethylase, deoxyCMP hydroxymethylase, deoxycytidylate hydroxymethylase, and deoxycytidylic hydroxymethylase. This enzyme participates in pyrimidine metabolism
Pyrimidine metabolism
Pyrimidine biosynthesis occurs both in the body and through organic synthesis.-De novo biosynthesis of pyrimidine :Unlike purines, pyrimidines are assembled before being attached to 5-phosphoribosyl-1-pyrophosphate ....
and one carbon pool by folate.
Structural studies
As of late 2007, 3 structuresTertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
have been solved for this class of enzymes, with PDB
Protein Data Bank
The Protein Data Bank is a repository for the 3-D structural data of large biological molecules, such as proteins and nucleic acids....
accession codes , , and .

