
Cottrell equation
Encyclopedia
In electrochemistry
, the Cottrell equation describes the change in electric current
with respect to time in a controlled potential experiment, such as chronoamperometry
. Specifically it describes the current response when the potential is a step function. It was derived by Frederick Gardner Cottrell
in 1903. For a simple redox
event, such as the ferrocene
/ferrocenium couple, the current measured depends on the rate at which the analyte
diffuses to the electrode. That is, the current is said to be "diffusion controlled." The Cottrell equation describes the case for an electrode that is planar but can also be derived for spherical, cylindrical, and rectangular geometries by using the corresponding laplace operator
and boundary conditions in conjunction with Fick's second law of diffusion.
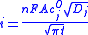
Where
Deviations from linearity in the plot of i vs t-1/2 sometimes indicate that the redox event is associated with other processes, such as association of a ligand
, dissociation of a ligand, or a change in geometry.
In practice, the Cottrell equation simplifies to
Furthermore, (scan rate)1/2 is used in place of t-1/2. Typical scan rates are in the range 20 to 2000 mV/s.
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
, the Cottrell equation describes the change in electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
with respect to time in a controlled potential experiment, such as chronoamperometry
Chronoamperometry
Chronoamperometry is an electrochemical technique in which the potential of the working electrode is stepped and the resulting current from faradic processes occurring at the electrode is monitored as a function of time...
. Specifically it describes the current response when the potential is a step function. It was derived by Frederick Gardner Cottrell
Frederick Gardner Cottrell
Frederick Gardner Cottrell was an American physical chemist and inventor. A native of Oakland, California, his immense curiosity gained him notice as a prodigious reader. But his achievements were also an ambitious response to economic necessity...
in 1903. For a simple redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
event, such as the ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
/ferrocenium couple, the current measured depends on the rate at which the analyte
Analyte
An analyte, or component , is a substance or chemical constituent that is of interest in an analytical procedure. Grammatically, it is important to note that experiments always seek to measure properties of analytes—and that analytes themselves can never be measured. For instance, one cannot...
diffuses to the electrode. That is, the current is said to be "diffusion controlled." The Cottrell equation describes the case for an electrode that is planar but can also be derived for spherical, cylindrical, and rectangular geometries by using the corresponding laplace operator
Laplace's equation
In mathematics, Laplace's equation is a second-order partial differential equation named after Pierre-Simon Laplace who first studied its properties. This is often written as:where ∆ = ∇² is the Laplace operator and \varphi is a scalar function...
and boundary conditions in conjunction with Fick's second law of diffusion.
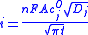
Where
- i = current, in unit A
- n = number of electrons (to reduce/oxidize one molecule of analyte j, for example)
- F = Faraday constant, 96,485 C/mol
- A = area of the (planar) electrode in cm2
- cjO = initial concentration of the reducible analyte j in mol/cm3;
- Dj = diffusion coefficientFick's law of diffusionFick's laws of diffusion describe diffusion and can be used to solve for the diffusion coefficient, D. They were derived by Adolf Fick in the year 1855.- Fick's first law :...
for species j in cm2/s - t = time in s
Deviations from linearity in the plot of i vs t-1/2 sometimes indicate that the redox event is associated with other processes, such as association of a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
, dissociation of a ligand, or a change in geometry.
In practice, the Cottrell equation simplifies to
- i = kt-1/2, where k is the collection of constants for a given system (n, F, A, coO, DO).
Furthermore, (scan rate)1/2 is used in place of t-1/2. Typical scan rates are in the range 20 to 2000 mV/s.
See also
- VoltammetryVoltammetryVoltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied.- Three electrode system :...
- Electroanalytical Methods
- Sand Equation
- ChronoamperometryChronoamperometryChronoamperometry is an electrochemical technique in which the potential of the working electrode is stepped and the resulting current from faradic processes occurring at the electrode is monitored as a function of time...

