
Chemical ionization
Encyclopedia
Chemical ionization is an ionization
technique used in mass spectrometry
. Chemical ionization is a lower energy process than electron ionization
. The lower energy yields less fragmentation, and usually a simpler spectrum
. A typical CI spectra has an easily identifiable molecular ion.
. Some common reagent gases include: methane
, ammonia
, and isobutane
. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source will preferentially ionize the reagent gas. The resultant collisions with other reagent gas molecules will create an ionization plasma
. Positive and negative ions of the analyte are formed by reactions with this plasma.
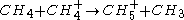
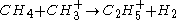
 (protonation)
(protonation)
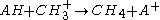 (
(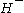 abstraction)
abstraction)
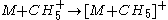 (adduct formation)
(adduct formation)
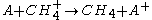 (charge exchange
(charge exchange
)
Self chemical ionization occurs when the reagent ion is an ionized form of the analyte.
In order to see a response by negative chemical ionization, the analyte must be capable of producing a negative ion (stabilize a negative charge) for example by electron capture ionization. Because not all analytes can do this, using NCI provides a certain degree of selectivity that is not available with other, more universal ionization techniques (EI, PCI). NCI can be used for the analysis of compounds containing acidic groups or electronegative elements (especially halogens).
Because of the high electronegativity of halogen
atoms, NCI is a common choice for their analysis. This includes many groups of compounds, such as PCBs, pesticides, and fire retardants. Most of these compounds are environmental contaminants, thus much of the NCI analysis that takes place is done under the auspices of environmental analysis. In cases where very low limits of detection are needed, halogenated species are frequently analyzed using an electron capture detector
coupled to a gas chromatograph.
. The analyte is a gas or liquid spray and ionization is accomplished using an atmospheric pressure corona discharge. This ionization method is often coupled with high performance liquid chromatography
where the mobile phase containing eluting analyte sprayed with high flow rates of nitrogen and the aerosol spray is subjected to a corona discharge to create ions.
Ionization
Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
technique used in mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
. Chemical ionization is a lower energy process than electron ionization
Electron ionization
Electron ionization is an ionization method in which energetic electrons interact with gas phase atoms or molecules to produce ions...
. The lower energy yields less fragmentation, and usually a simpler spectrum
Spectrum
A spectrum is a condition that is not limited to a specific set of values but can vary infinitely within a continuum. The word saw its first scientific use within the field of optics to describe the rainbow of colors in visible light when separated using a prism; it has since been applied by...
. A typical CI spectra has an easily identifiable molecular ion.
Mechanism
In a CI experiment, ions are produced through the collision of the analyte with ions of a reagent gas that are present in the ion sourceIon source
An ion source is an electro-magnetic device that is used to create charged particles. These are used primarily to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.- Electron ionization :...
. Some common reagent gases include: methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, and isobutane
Isobutane
Isobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,...
. Inside the ion source, the reagent gas is present in large excess compared to the analyte. Electrons entering the source will preferentially ionize the reagent gas. The resultant collisions with other reagent gas molecules will create an ionization plasma
Plasma (physics)
In physics and chemistry, plasma is a state of matter similar to gas in which a certain portion of the particles are ionized. Heating a gas may ionize its molecules or atoms , thus turning it into a plasma, which contains charged particles: positive ions and negative electrons or ions...
. Positive and negative ions of the analyte are formed by reactions with this plasma.
Secondary reagent ions
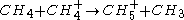
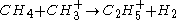
Product ion formation
 (protonation)
(protonation)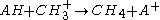 (
(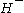 abstraction)
abstraction)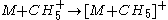 (adduct formation)
(adduct formation)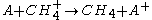 (charge exchange
(charge exchangeCharge-exchange ionization
Charge-exchange ionization is a gas phase reaction between an ion and an atom or molecule in which the charge of the ion is transferred to the neutral species.For exampleA^+ + B \to A + B^+....
)
Self chemical ionization occurs when the reagent ion is an ionized form of the analyte.
Negative chemical ionization (NCI)
Chemical ionization for gas phase analysis is either positive or negative. Almost all neutral analytes can form positive ions through the reactions described above.In order to see a response by negative chemical ionization, the analyte must be capable of producing a negative ion (stabilize a negative charge) for example by electron capture ionization. Because not all analytes can do this, using NCI provides a certain degree of selectivity that is not available with other, more universal ionization techniques (EI, PCI). NCI can be used for the analysis of compounds containing acidic groups or electronegative elements (especially halogens).
Because of the high electronegativity of halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
atoms, NCI is a common choice for their analysis. This includes many groups of compounds, such as PCBs, pesticides, and fire retardants. Most of these compounds are environmental contaminants, thus much of the NCI analysis that takes place is done under the auspices of environmental analysis. In cases where very low limits of detection are needed, halogenated species are frequently analyzed using an electron capture detector
Electron capture detector
An electron capture detector is a device for detecting atoms and molecules in a gas through the attachment of electrons via electron capture ionization. The device was invented in 1957 by Dr. James E...
coupled to a gas chromatograph.
Atmospheric pressure chemical ionization (APCI)
Chemical ionization in an atmospheric pressure electric discharge is called atmospheric pressure chemical ionizationAtmospheric pressure chemical ionization
Atmospheric-pressure chemical ionization is an ionization method used in mass spectrometry. It is a form of chemical ionization which takes place at atmospheric pressure.-How it works:...
. The analyte is a gas or liquid spray and ionization is accomplished using an atmospheric pressure corona discharge. This ionization method is often coupled with high performance liquid chromatography
High performance liquid chromatography
High-performance liquid chromatography , HPLC, is a chromatographic technique that can separate a mixture of compounds and is used in biochemistry and analytical chemistry to identify, quantify and purify the individual components of the mixture.HPLC typically utilizes different types of stationary...
where the mobile phase containing eluting analyte sprayed with high flow rates of nitrogen and the aerosol spray is subjected to a corona discharge to create ions.

