Carroll rearrangement
Encyclopedia
The Carroll rearrangement is a rearrangement reaction
in organic chemistry
and involves the transformation of a β-keto
allyl
ester
into a α-allyl-β-ketocarboxylic acid. This organic reaction
is accompanied by decarboxylation
and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement
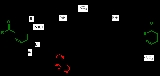
and effectively a decarboxylative Allylation.
and with high reaction temperature (path A) takes place through an intermediate enol
which then rearranges in an electrocyclic Claisen rearrangement
. The follow-up is a decarboxylation. With palladium(0)
as a catalyst, the reaction (Tsuji, 1980) is much milder (path B) with an intermediate allyl cation / carboxylic acid anion organometallic complex.
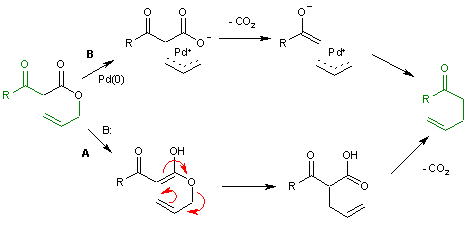 Decarboxylation precedes allylation as evidenced by this reaction catalyzed by tetrakis(triphenylphosphine)palladium(0)
Decarboxylation precedes allylation as evidenced by this reaction catalyzed by tetrakis(triphenylphosphine)palladium(0)
:
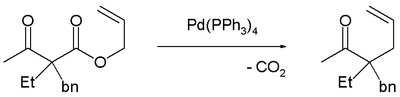
The first reported asymmetric rearrangement is catalyzed by tris(dibenzylideneacetone)dipalladium(0)
and the Trost ligand
:
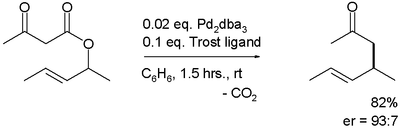 A similar reaction uses additional naphthol
A similar reaction uses additional naphthol
.
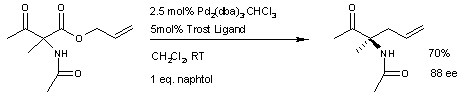 This reaction delivers one enantiomer
This reaction delivers one enantiomer
with 88% ee
. It remains to be seen if this reaction will have a wide scope because the acetamido group appears to be a prerequisite.
The same catalyst but a different ligand is employed in this enantioconvergent reaction :
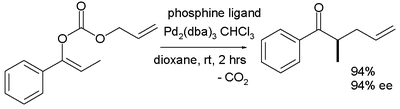
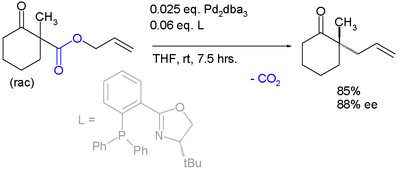 The scope is extended to asymmetric α-alkylation of ketone
The scope is extended to asymmetric α-alkylation of ketone
s masked as their enol carbonate ester
s :
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and involves the transformation of a β-keto
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
into a α-allyl-β-ketocarboxylic acid. This organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
is accompanied by decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement
Claisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
and effectively a decarboxylative Allylation.
Reaction mechanism
The Carroll rearrangement (1940) in the presence of baseBase (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
and with high reaction temperature (path A) takes place through an intermediate enol
Enol
Enols are alkenes with a hydroxyl group affixed to one of the carbon atoms composing the double bond. Alkenes with a hydroxyl group on both sides of the double bond are called enediols. Deprotonated anions of enols are called enolates...
which then rearranges in an electrocyclic Claisen rearrangement
Claisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
. The follow-up is a decarboxylation. With palladium(0)
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
as a catalyst, the reaction (Tsuji, 1980) is much milder (path B) with an intermediate allyl cation / carboxylic acid anion organometallic complex.

Tetrakis(triphenylphosphine)palladium(0)
Tetrakispalladium is the chemical compound Pd[P3]4, often abbreviated Pd4, or even PdP4. It is a bright yellow crystalline solid that becomes brown upon decomposition in air.-Structure and properties:...
:

Asymmetric decarboxylative allylation
By introducing suitable chiral ligands, the reaction becomes enantioselective.The first reported asymmetric rearrangement is catalyzed by tris(dibenzylideneacetone)dipalladium(0)
Tris(dibenzylideneacetone)dipalladium(0)
Trisdipalladium or Pd23 is an organometallic complex based on palladium and dibenzylideneacetone used in organic chemistry. It was discovered in 1970.-Preparation and structure:...
and the Trost ligand
Trost ligand
The Trost ligand is a chiral ligand pioneered by Barry Trost for use in the palladium-catalyzed Trost asymmetric allylic alkylation. Other C2-symmetric ligands derived from trans-1,2-diaminocyclohexane have also been developed by the Trost group, such as the -DACH-naphthyl ligand derived from...
:

Naphthol
Naphthol may refer to:* 1-Naphthol* 2-Naphthol...
.
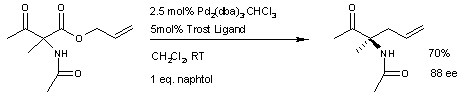
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
with 88% ee
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
. It remains to be seen if this reaction will have a wide scope because the acetamido group appears to be a prerequisite.
The same catalyst but a different ligand is employed in this enantioconvergent reaction :

Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s masked as their enol carbonate ester
Carbonate ester
A carbonate ester is a functional group in organic chemistry consisting of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1OOR2 and they are related to esters R1OR and ethers R1OR2 and also to the inorganic carbonates.Carbonate esters are used as...
s :


