
Butler-Volmer equation
Encyclopedia
The Butler–Volmer equation is one of the most fundamental relationships in electrochemical kinetics
. It describes how the electrical current on an electrode depends on the electrode potential, considering that both a cathodic and an anodic reaction occur on the same electrode:
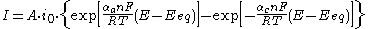
or in a more compact form:
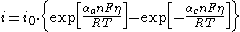
where:
The equation is named after chemists John Alfred Valentine Butler
and Max Volmer
.
In the region of the limiting current
, when the electrode process is mass-transfer
controlled, the value of the current density is: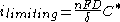
where:
The more general form of the Butler–Volmer equation, applicable to the mass transfer-influenced conditions, can be written as:
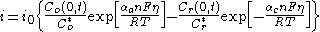
where:
The above form simplifies to the conventional one (shown at the top of the article) when the concentration of the electroactive species at the surface equals to that in the bulk.
where a and b are constants (for a given reaction and temperature) and are called the Tafel equation constants. The theoretical values of a and b are different for the cathodic and anodic processes.
Electrochemical kinetics
Electrochemical kinetics is a field of electrochemistry studying the rate of electrochemical processes. Due to electrochemical phenomena unfolding at the interface between an electrode and an electrolyte, there are accompanying phenomena to electrochemical reaction which contribute to the overall...
. It describes how the electrical current on an electrode depends on the electrode potential, considering that both a cathodic and an anodic reaction occur on the same electrode:
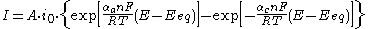
or in a more compact form:
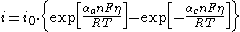
where:
-
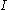 : electrode current, A
: electrode current, A -
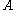 : electrode active surface area, m2
: electrode active surface area, m2 -
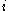 : electrode current densityCurrent densityCurrent density is a measure of the density of flow of a conserved charge. Usually the charge is the electric charge, in which case the associated current density is the electric current per unit area of cross section, but the term current density can also be applied to other conserved...
: electrode current densityCurrent densityCurrent density is a measure of the density of flow of a conserved charge. Usually the charge is the electric charge, in which case the associated current density is the electric current per unit area of cross section, but the term current density can also be applied to other conserved...
, A/m2 (defined as i = I/A) -
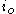 : exchange current densityExchange current densityIn electrochemistry, exchange current density is a parameter used in the Tafel equation, Butler-Volmer equation and other expressions. The Tafel equation describes the dependence of current for an electrolytic process to overpotential....
: exchange current densityExchange current densityIn electrochemistry, exchange current density is a parameter used in the Tafel equation, Butler-Volmer equation and other expressions. The Tafel equation describes the dependence of current for an electrolytic process to overpotential....
, A/m2 -
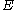 : electrode potentialElectrode potentialElectrode potential, E, in electrochemistry, according to an IUPAC definition, is the electromotive force of a cell built of two electrodes:* on the left-hand side is the standard hydrogen electrode, and...
: electrode potentialElectrode potentialElectrode potential, E, in electrochemistry, according to an IUPAC definition, is the electromotive force of a cell built of two electrodes:* on the left-hand side is the standard hydrogen electrode, and...
, V -
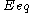 : equilibrium potential, V
: equilibrium potential, V -
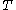 : absolute temperature, K
: absolute temperature, K -
 : number of electrons involved in the electrode reaction
: number of electrons involved in the electrode reaction -
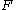 : Faraday constant
: Faraday constant -
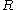 : universal gas constant
: universal gas constant -
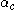 : so-called cathodic charge transfer coefficientCharge transfer coefficientCharge transfer coefficient, and symmetry factor are two related parameters used in description of the kinetics of electrochemical reactions...
: so-called cathodic charge transfer coefficientCharge transfer coefficientCharge transfer coefficient, and symmetry factor are two related parameters used in description of the kinetics of electrochemical reactions...
, dimensionless -
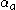 : so-called anodic charge transfer coefficient, dimensionless
: so-called anodic charge transfer coefficient, dimensionless -
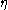 : activation overpotentialOverpotentialOverpotential is an electrochemical term which refers to the potential difference between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency...
: activation overpotentialOverpotentialOverpotential is an electrochemical term which refers to the potential difference between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency...
(defined as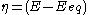 ).
).
The equation is named after chemists John Alfred Valentine Butler
John Alfred Valentine Butler
John Alfred Valentine Butler was an English physical chemist best known for his contributions to the development of electrode kinetics .-Works:* The Fundamentals of Chemical Thermodynamics ....
and Max Volmer
Max Volmer
Max Volmer was a German physical chemist, who made important contributions in electrochemistry, in particular on electrode kinetics. He co-developed the Butler–Volmer equation. Volmer held the chair and directorship of the Physical Chemistry and Electrochemistry Institute of the Technische...
.
Mass-transfer control
The previous form of Butler–Volmer equation is valid when the electrode reaction is controlled by electrical charge transfer at the electrode (and not by the mass transfer to or from the electrode surface from or to the bulk electrolyte). Nevertheless, the utility of the Butler–Volmer equation in electrochemistry is wide, and it is often considered to be "central in the phenomenological electrode kinetics".In the region of the limiting current
Limiting current
The limiting current, in electrochemistry, is the limiting value of a faradaic current that is approached as the rate of charge-transfer to an electrode is increased . The limiting current can be approached, for example, by increasing the electric potential or decreasing the rate of mass transfer...
, when the electrode process is mass-transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
controlled, the value of the current density is:
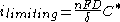
where:
- D is the diffusion coefficient;
- δ is the diffusion layer thickness;
- C* is the concentration of the electroactive (limiting) species in the bulk of the electrolyte.
The more general form of the Butler–Volmer equation, applicable to the mass transfer-influenced conditions, can be written as:
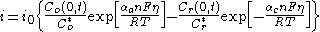
where:
- i is the current density, A/m2,
- Co and Cr refer to the concentration of the oxidized and the reduced form, respectively,
- C(0,t) is the time-dependent concentration at the distance zero from the surface.
The above form simplifies to the conventional one (shown at the top of the article) when the concentration of the electroactive species at the surface equals to that in the bulk.
The limiting cases
There are two limiting cases of the Butler–Volmer equation:- the low overpotential region (called "polarization resistance", i.e., when E ≈ Eeq), where the Butler–Volmer equation simplifies to:
-
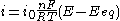 ;
;
- the high overpotential region, where the Butler–Volmer equation simplifies to the Tafel equationTafel equationThe Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification...
:
-
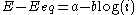 for a cathodic reaction, when E << Eeq, or
for a cathodic reaction, when E << Eeq, or -
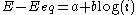 for an anodic reaction, when E >> Eeq
for an anodic reaction, when E >> Eeq
where a and b are constants (for a given reaction and temperature) and are called the Tafel equation constants. The theoretical values of a and b are different for the cathodic and anodic processes.
See also
- Nernst equationNernst equationIn electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
- Tafel equationTafel equationThe Tafel equation is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced experimentally and was later shown to have a theoretical justification...
- Goldman equationGoldman equationThe Goldman–Hodgkin–Katz voltage equation, more commonly known as the Goldman equation is used in cell membrane physiology to determine the equilibrium potential across a cell's membrane taking into account all of the ions that are permeant through that membrane.The discoverers of this are David E...
- Max VolmerMax VolmerMax Volmer was a German physical chemist, who made important contributions in electrochemistry, in particular on electrode kinetics. He co-developed the Butler–Volmer equation. Volmer held the chair and directorship of the Physical Chemistry and Electrochemistry Institute of the Technische...
- John Alfred Valentine ButlerJohn Alfred Valentine ButlerJohn Alfred Valentine Butler was an English physical chemist best known for his contributions to the development of electrode kinetics .-Works:* The Fundamentals of Chemical Thermodynamics ....

