
Brusselator
Encyclopedia
The Brusselator is a theoretical model for a type of autocatalytic reaction
.
The Brusselator model was proposed by Ilya Prigogine
and his collaborators at the Free University of Brussels
.
It is a portmanteau of Brussels
and oscillator.
It is characterized by the reactions
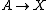
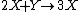
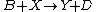
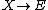
with the rate equations
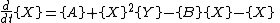
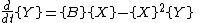
where, for convenience, the rate constants have been set to 1.
The Brusselator has a fixed point at
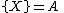
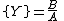 .
.
The fixed point becomes unstable when
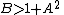
leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.
The best-known example is the clock reaction, the Belousov-Zhabotinsky reaction
(BZ reaction). It can be created with a mixture of potassium bromate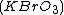 , malonic acid
, malonic acid 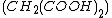 , and manganese sulfate
, and manganese sulfate 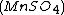 prepared in a heated solution of sulfuric acid
prepared in a heated solution of sulfuric acid 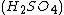 .
.
Autocatalytic reaction
Autocatalytic reactions are chemical reactions in which at least one of the reactants is also a product. The rate equations for autocatalytic reactions are fundamentally nonlinear. This nonlinearity can lead to the spontaneous generation of order. A dramatic example of this order is that which is...
.
The Brusselator model was proposed by Ilya Prigogine
Ilya Prigogine
Ilya, Viscount Prigogine was a Russian-born naturalized Belgian physical chemist and Nobel Laureate noted for his work on dissipative structures, complex systems, and irreversibility.-Biography :...
and his collaborators at the Free University of Brussels
Free University of Brussels
The Free University of Brussels was a university in Brussels, Belgium. In 1969, it split into the Université Libre de Bruxelles and the Dutch-speaking Vrije Universiteit Brussel....
.
It is a portmanteau of Brussels
Brussels
Brussels , officially the Brussels Region or Brussels-Capital Region , is the capital of Belgium and the de facto capital of the European Union...
and oscillator.
It is characterized by the reactions
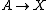
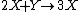
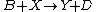
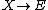
with the rate equations
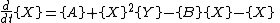
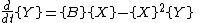
where, for convenience, the rate constants have been set to 1.
The Brusselator has a fixed point at
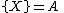
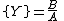 .
.The fixed point becomes unstable when
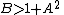
leading to an oscillation of the system. Unlike the Lotka-Volterra equation, the oscillations of the Brusselator do not depend on the amount of reactant present initially. Instead, after sufficient time, the oscillations approach a limit cycle.
The best-known example is the clock reaction, the Belousov-Zhabotinsky reaction
Belousov-Zhabotinsky reaction
A Belousov–Zhabotinsky reaction, or BZ reaction, is one of a class of reactions that serve as a classical example of non-equilibrium thermodynamics, resulting in the establishment of a nonlinear chemical oscillator. The only common element in these oscillating systems is the inclusion of bromine...
(BZ reaction). It can be created with a mixture of potassium bromate
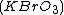 , malonic acid
, malonic acid 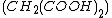 , and manganese sulfate
, and manganese sulfate 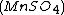 prepared in a heated solution of sulfuric acid
prepared in a heated solution of sulfuric acid 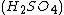 .
.

