
Bismuth(III) sulfide
Encyclopedia
Bismuth sulfide is a chemical compound
of bismuth
and sulfur
. It occurs in nature as the mineral bismuthinite
.
with hydrogen sulfide
:
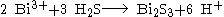
Bismuth (III) sulfide can also be prepared by the reaction of elemental bismuth and elemental sulfur in an evacuated silica tube at 500 °C for 96 hours.
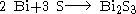
It can react with acids to produce the odoriferous hydrogen sulfide
gas.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
of bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. It occurs in nature as the mineral bismuthinite
Bismuthinite
Bismuthinite is a mineral consisting of bismuth sulfide . It is an important ore for bismuth. The crystals are steel-grey to off-white with a metallic luster...
.
Synthesis
Bismuth(III) sulfide can be prepared by reacting a bismuth(III) saltSalt
In chemistry, salts are ionic compounds that result from the neutralization reaction of an acid and a base. They are composed of cations and anions so that the product is electrically neutral...
with hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
:
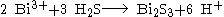
Bismuth (III) sulfide can also be prepared by the reaction of elemental bismuth and elemental sulfur in an evacuated silica tube at 500 °C for 96 hours.
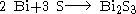
Properties
Bismuth(III) sulfide has a trigonal prismatic crystal structure.It can react with acids to produce the odoriferous hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
gas.

