Berry mechanism
Encyclopedia
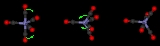
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation
. It most commonly occurs in trigonal bipyramidal molecules, such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry
.
where the base is the four interchanging ligands and the tip is the pivot ligand, which has not moved. The two originally equatorial ligands then open out until they are 180 degrees apart, becoming axial groups perpendicular to where the axial groups were before the pseudorotation.
 This rapid exchange of axial and equatorial ligands renders complexes with this geometry unresolvable (unlike carbon atoms with four distinct substituents), except at low temperatures or when one or more of the ligands is bi- or poly-dentate.
This rapid exchange of axial and equatorial ligands renders complexes with this geometry unresolvable (unlike carbon atoms with four distinct substituents), except at low temperatures or when one or more of the ligands is bi- or poly-dentate.
) is somewhat like the inverse of the mechanism in bipyramidal molecules. Starting at the "transition phase" of bipyramidal pseudorotation, one pair of fluorines scissors back and forth with a third fluorine, causing the molecule to vibrate. Unlike with pseudorotation in bipyramidal molecules, the atoms and ligands which are not actively vibrating in the "scissor" motion are still participating in the process of pseudorotation; they make general adjustment based on the movement of the actively vibrating atoms and ligands.
Pseudorotation
The IUPAC defines pseudorotation as "a conformational change resulting in a structure that appears to have been produced by rotation of the entire initial molecule and is superimposable on the initial one, unless different positions are distinguished by substitution or isotopic labeling...
. It most commonly occurs in trigonal bipyramidal molecules, such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry
R. Stephen Berry
R. Stephen Berry is a U.S. professor of physical chemistry.He is the James Franck Distinguished Service Professor Emeritus at The University of Chicago and Special Advisor to the Director for National Security, at Argonne National Laboratory...
.
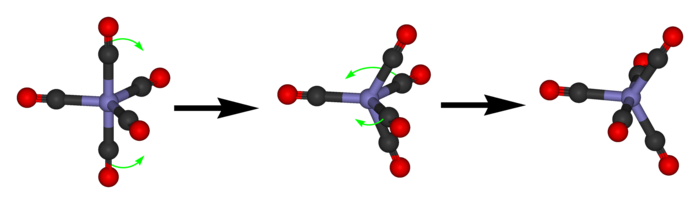
Berry mechanism in trigonal bipyramidal structure
The process of pseudorotation occurs when the two axial ligands close like a pair of scissors pushing their way in between two of the equatorial groups which scissor out to accommodate them. This forms a square based pyramidSquare pyramidal molecular geometry
In molecular geometry, square based pyramidal geometry describes the shape of certain compounds with the formula ML5 where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The geometry is common for certain main group compounds...
where the base is the four interchanging ligands and the tip is the pivot ligand, which has not moved. The two originally equatorial ligands then open out until they are 180 degrees apart, becoming axial groups perpendicular to where the axial groups were before the pseudorotation.

Berry mechanism in square pyramidal structure
The Berry mechanism in square pyramidal molecules (such as IF5Iodine pentafluoride
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is a fluoride of iodine. It is a colourless or yellow liquid with a density of 3.250 g cm−3. It was first synthesized by Henri Moissan in 1891 by burning solid iodine in fluorine gas...
) is somewhat like the inverse of the mechanism in bipyramidal molecules. Starting at the "transition phase" of bipyramidal pseudorotation, one pair of fluorines scissors back and forth with a third fluorine, causing the molecule to vibrate. Unlike with pseudorotation in bipyramidal molecules, the atoms and ligands which are not actively vibrating in the "scissor" motion are still participating in the process of pseudorotation; they make general adjustment based on the movement of the actively vibrating atoms and ligands.

