Pseudorotation
Encyclopedia
The IUPAC defines pseudorotation as "a conformational change resulting in a structure that appears to have been produced by rotation of the entire initial molecule and is superimposable on the initial one, unless different positions are distinguished by substitution or isotopic labeling. No angular momentum is generated by this motion; this is the reason for the term." Formally it refers to symmetrical molecules, although the mechanism is invoked for low-symmetry species also. A small displacement of the atomic positions leads to a loss of symmetry until the symmetric product forms. Although not formally required, pseudorotation normally involves displacements along low-energy pathways.
refers to the facile interconversion of axial and equatorial ligand in MX5 compounds, e.g. D3h-symmetric PF5:
MX5 compounds
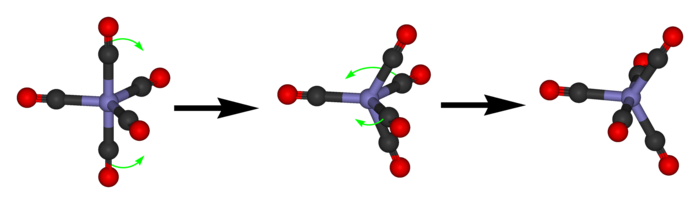
The Berry mechanismBerry mechanism
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation...
refers to the facile interconversion of axial and equatorial ligand in MX5 compounds, e.g. D3h-symmetric PF5:


