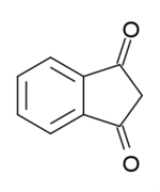
1,3-Indandione
Encyclopedia
1,3-indandione is an aromatic trans-fixed β-diketone
, in standard conditions it is referred to in different sources as colourless or yellowish, green or (most commonly) yellow solid. It is an anticoagulant
used as a rodenticide.
A valuable contribution in research of 1,3-indandiones and other diketones belongs to a great Latvian chemist Gustavs Vanags.
of ethyl acetate and diethyl phthalate (of course another alcohol moieties can be present in the raw materials).
. It undergoes self-condensation quite easily, resulting in bindone.
Another example is bromination.
1,3-indandione could be reduced till indanone, 3-hydroxy-1-insanone, 1,3-indanediol or even indane, depending on the method used.
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
, in standard conditions it is referred to in different sources as colourless or yellowish, green or (most commonly) yellow solid. It is an anticoagulant
Anticoagulant
An anticoagulant is a substance that prevents coagulation of blood. A group of pharmaceuticals called anticoagulants can be used in vivo as a medication for thrombotic disorders. Some anticoagulants are used in medical equipment, such as test tubes, blood transfusion bags, and renal dialysis...
used as a rodenticide.
A valuable contribution in research of 1,3-indandiones and other diketones belongs to a great Latvian chemist Gustavs Vanags.
Structural properties
In the solid 1,3-indandione occurs as a diketone whereas its water solution is partially (~2%) enolized. The enolate anion exhibits significant delocalisation, and the highest electron density is on the second carbon. This explains many of chemical properties of the compound.Preparation
The well-known method is decarboxilation of the sodium salt of 2-etoxycarbonyl-1,3-indandione, which itself is obtained by Claisen condensationClaisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
of ethyl acetate and diethyl phthalate (of course another alcohol moieties can be present in the raw materials).
Chemical properties
1,3-Indandione is a very strong C-nucleophileNucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
. It undergoes self-condensation quite easily, resulting in bindone.
Another example is bromination.
1,3-indandione could be reduced till indanone, 3-hydroxy-1-insanone, 1,3-indanediol or even indane, depending on the method used.

