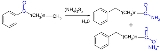
Willgerodt rearrangement
Encyclopedia
The Willgerodt rearrangement or Willgerodt reaction is an organic reaction
converting an aryl alkyl ketone
to the corresponding amide
by reaction with ammonium polysulfide
, named after Conrad Willgerodt
. The formation of the corresponding carboxylic acid
is a side reaction. When the alkyl group is an aliphatic chain (n typically 0 to 5), multiple reactions take place with the amide group always ending up at the terminal end.
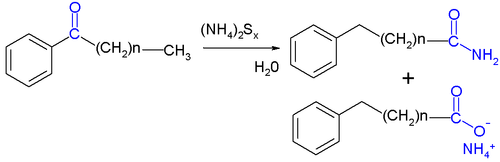 An example with modified reagents (sulfur, concentrated ammonium hydroxide
An example with modified reagents (sulfur, concentrated ammonium hydroxide
and pyridine
) is the conversion of acetophenone
to 2-phenylacetamide and phenylacetic acid
:
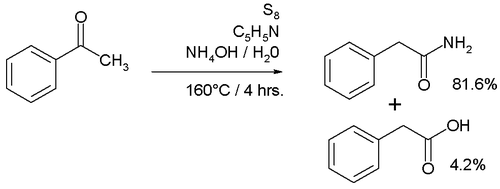
and an amine
like morpholine
. The initial product is a thioacetamide
for example that of acetophenone
which can again be hydrolyzed to the amide. The reaction is named after Karl Kindler.
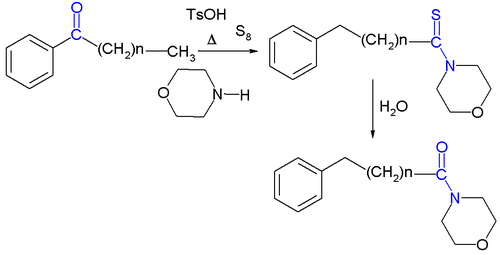
for the Kindler variation is depicted below:
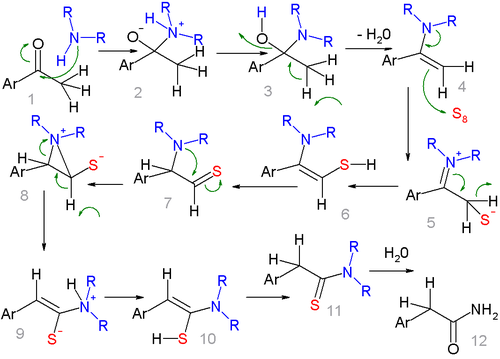 The first stage of the reaction is basic imine formation
The first stage of the reaction is basic imine formation
by the ketone
group and the amine
group of morpholine to the enamine
4 which reacts in a conjugate addition (see Stork enamine alkylation
for a related step) with sulfur to the sulfide
6. The actual rearrangement reaction
takes place when the amine group attacks thiocarbonyl 7 in a nucleophilic addition
temporarily forming an aziridine
ring (8) and the amine group moving along the central C-C bond to 9 and after proton exchange to 10 and further on to the thioacetamide
11 by tautomerization.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
converting an aryl alkyl ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
to the corresponding amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
by reaction with ammonium polysulfide
Polysulfide
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: anions and organic polysulfides. Anions have the general formula Sn2−. These anions are the conjugate bases of the hydrogen polysulfides H2nSn...
, named after Conrad Willgerodt
Conrad Willgerodt
Conrad Heinrich Christoph Willgerodt was a German chemist and discovered of the Willgerodt reaction. He was also the discoverer of Iodosobenzene.Willgerodt was a professor at the University of Freiburg.-References:...
. The formation of the corresponding carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
is a side reaction. When the alkyl group is an aliphatic chain (n typically 0 to 5), multiple reactions take place with the amide group always ending up at the terminal end.

Ammonium hydroxide
Ammonia solution, also known as ammonium hydroxide, ammonia water, ammonical liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or simply ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3...
and pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
) is the conversion of acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
to 2-phenylacetamide and phenylacetic acid
Phenylacetic acid
Phenylacetic acid is an organic compound containing a phenyl functional group and a carboxylic acid functional group. It is a white solid with a disagreeable odor...
:

Willgerodt-Kindler reaction
The related Willgerodt-Kindler reaction takes place with elemental sulfurSulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
like morpholine
Morpholine
Morpholine is an organic chemical compound having the chemical formula O2NH. This heterocycle, pictured at right, features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium...
. The initial product is a thioacetamide
Thioacetamide
Thioacetamide is an organosulfur compound with the formula C2H5NS. This white crystalline solid is soluble in water and serves as a source of sulfide ions in the synthesis of organic and inorganic compounds. It is a prototypical thioamide....
for example that of acetophenone
Acetophenone
Acetophenone is the organic compound with the formula C6H5CCH3. It is the simplest aromatic ketone. This colourless, viscous liquid is a precursor to useful resins and fragrances.-Production:Acetophenone can be obtained by a variety of methods...
which can again be hydrolyzed to the amide. The reaction is named after Karl Kindler.

Reaction mechanism
A possible reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
for the Kindler variation is depicted below:

Alkylimino-de-oxo-bisubstitution
Alkylimino-de-oxo-bisubstitution in organic chemistry is the organic reaction of carbonyl compounds with amines to imines . The reaction name is based on the IUPAC Nomenclature for Transformations...
by the ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
group and the amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
group of morpholine to the enamine
Enamine
An enamine is an unsaturated compound derived by the reaction of an aldehyde or ketone with a secondary amine followed by loss of H2O.The word "enamine" is derived from the affix en-, used as the suffix of alkene, and the root amine. This can be compared with enol, which is a functional group...
4 which reacts in a conjugate addition (see Stork enamine alkylation
Stork enamine alkylation
Stork enamine alkylation, also known as the Stork-Enamine reaction, involves the addition of an enamine to an alpha, beta-unsaturated carbonyl acceptor in a process similar to the Michael reaction...
for a related step) with sulfur to the sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
6. The actual rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
takes place when the amine group attacks thiocarbonyl 7 in a nucleophilic addition
Nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile....
temporarily forming an aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
ring (8) and the amine group moving along the central C-C bond to 9 and after proton exchange to 10 and further on to the thioacetamide
Thioacetamide
Thioacetamide is an organosulfur compound with the formula C2H5NS. This white crystalline solid is soluble in water and serves as a source of sulfide ions in the synthesis of organic and inorganic compounds. It is a prototypical thioamide....
11 by tautomerization.

