
Ununennium
Encyclopedia
Ununennium also known as eka
-francium
or element 119, is the temporary name of a hypothetical chemical element
in the periodic table
that has the temporary symbol Uue and has the atomic number
119. To date, attempted syntheses of this element have been unsuccessful. Since it is below the alkali metals it might have properties similar to those of francium
or caesium
. Like other alkali metals, it should be extremely reactive with water and air. A predicted oxidation state is +1. Ununennium would be the first element in the eighth period
of the periodic table
and possibly the seventh alkali metal, though relativistic effects might make it less reactive than francium and caesium.
-254 with calcium
-48 ions at the superHILAC accelerator at Berkeley, California. No atoms were identified, leading to a limiting yield of 300 nb.
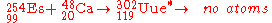
It is highly unlikely that this reaction will be useful given the extremely difficult task of making
sufficient amounts of 254Es to make a large enough target to increase the sensitivity of the experiment to the required level, due to the rarity of the element, and extreme rarity of the isotope.
DNS = Di-nuclear system; σ = cross section
Mendeleev's predicted elements
Professor Dmitri Mendeleev published the first Periodic Table of the Atomic Elements in 1869 based on properties which appeared with some regularity as he laid out the elements from lightest to heaviest....
-francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
or element 119, is the temporary name of a hypothetical chemical element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
in the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
that has the temporary symbol Uue and has the atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
119. To date, attempted syntheses of this element have been unsuccessful. Since it is below the alkali metals it might have properties similar to those of francium
Francium
Francium is a chemical element with symbol Fr and atomic number 87. It was formerly known as eka-caesium and actinium K.Actually the least unstable isotope, francium-223 It has the lowest electronegativity of all known elements, and is the second rarest naturally occurring element...
or caesium
Caesium
Caesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
. Like other alkali metals, it should be extremely reactive with water and air. A predicted oxidation state is +1. Ununennium would be the first element in the eighth period
Periodic table period
In the periodic table of the elements, elements are arranged in a series of rows so that those with similar properties appear in vertical columns. Elements of the same period have the same number of electron shells; with each group across a period, the elements have one more proton and electron...
of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
and possibly the seventh alkali metal, though relativistic effects might make it less reactive than francium and caesium.
Unsuccessful attempts at synthesis
The synthesis of ununennium was attempted in 1985 by bombarding a target of einsteiniumEinsteinium
Einsteinium is a synthetic element with the symbol Es and atomic number 99. It is the seventh transuranic element, and an actinide.Einsteinium was discovered in the debris of the first hydrogen bomb explosion in 1952, and named after Albert Einstein...
-254 with calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
-48 ions at the superHILAC accelerator at Berkeley, California. No atoms were identified, leading to a limiting yield of 300 nb.
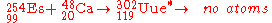
It is highly unlikely that this reaction will be useful given the extremely difficult task of making
sufficient amounts of 254Es to make a large enough target to increase the sensitivity of the experiment to the required level, due to the rarity of the element, and extreme rarity of the isotope.
Predicted decay characteristics
The alpha-decay half-lives of 1700 nuclei with 100 ≤ Z ≤ 130 have been calculated in a quantum tunneling model with alpha-decay Q-values from different mass estimates. The alpha-decay half-lives predicted for 291-307119 are of the order of micro-seconds. The highest value of the alpha-decay half-life predicted in the quantum tunneling model with the mass estimates from a macroscopic-microscopic model is ~485 microseconds for the isotope 294119. For 302119 it is ~163 microseconds.Target-projectile combinations leading to Z=119 compound nuclei
The below table contains various combinations of targets and projectiles which could be used to form compound nuclei with an atomic number of 119.| Target | Projectile | CN | Attempt result |
|---|---|---|---|
| 254Es | 48Ca | 302Uue |
Theoretical calculations on evaporation cross sections
The below table contains various targets-projectile combinations for which calculations have provided estimates for cross section yields from various neutron evaporation channels. The channel with the highest expected yield is given.DNS = Di-nuclear system; σ = cross section
| Target | Projectile | CN | Channel (product) | σ max | Model | Ref |
|---|---|---|---|---|---|---|
| 254Es | 48Ca | 302Uue | 3n (299Uue) | 0.5 pb | DNS |

