
Unrestricted Hartree-Fock
Encyclopedia
Unrestricted Hartree–Fock (UHF) theory is the most common molecular orbital method for open shell
molecules where the number of electrons of each spin are not equal. While restricted Hartree–Fock theory uses a single molecular orbital twice, once multiplied by the α spin function and once multiplied by the β spin function in the Slater determinant
, unrestricted Hartree–Fock theory uses different molecular orbitals for the α and β electrons. This has been called a different orbitals for different spins (DODS) method. The result is a pair of coupled Roothaan equations
, known as the Pople–Nesbet–Berthier equations.
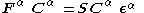
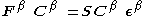
Where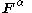 and
and 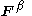 are the Fock matrices
are the Fock matrices
for the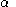 and
and 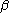 orbitals,
orbitals, 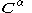 and
and 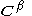 are the matrices of coefficients for the
are the matrices of coefficients for the  and
and 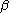 orbitals, S is the overlap matrix
orbitals, S is the overlap matrix
of the basis functions, and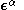 and
and 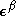 are the (diagonal, by convention) matrices of orbital energies for the
are the (diagonal, by convention) matrices of orbital energies for the 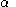 and
and 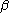 orbitals. The pair of equations are coupled because the Fock matrix elements of one spin contains coefficients of both spin as the orbital has to be optimized in the average field of all other electrons. The final result is a set of molecular orbitals and orbital energies for the α spin electrons and a set of molecular orbitals and orbital energies for the β electrons.
orbitals. The pair of equations are coupled because the Fock matrix elements of one spin contains coefficients of both spin as the orbital has to be optimized in the average field of all other electrons. The final result is a set of molecular orbitals and orbital energies for the α spin electrons and a set of molecular orbitals and orbital energies for the β electrons.
This method has one drawback. A single Slater determinant
of different orbitals for different spins is not a satisfactory eigenfunction of the total spin operator -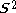 . The ground state is contaminated
. The ground state is contaminated
by excited states. If there is one more electron of α spin than β spin, the ground state is a doublet. The average value of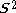 , written <
, written <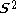 >, should be
>, should be 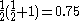 but will actually be rather more than this value as the doublet state is contaminated by a quadruplet state. A triplet state with two excess α electrons should have <
but will actually be rather more than this value as the doublet state is contaminated by a quadruplet state. A triplet state with two excess α electrons should have <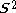 > = 1 (1 + 1) = 2, but it will be larger as the triplet is contaminated by a quintuplet state. When carrying out unrestricted Hartree–Fock calculations, it is always necessary to check this contamination. For example, with a doublet state, if <
> = 1 (1 + 1) = 2, but it will be larger as the triplet is contaminated by a quintuplet state. When carrying out unrestricted Hartree–Fock calculations, it is always necessary to check this contamination. For example, with a doublet state, if <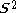 > = 0.8 or less, it is probably satisfactory. If it is 1.0 or so, it is certainly not satisfactory and the calculation should be rejected and a different approach taken. It requires experience to make this judgment. Even singlet states can suffer from spin-contamination, for example the H2 dissociation curve is discontinuous
> = 0.8 or less, it is probably satisfactory. If it is 1.0 or so, it is certainly not satisfactory and the calculation should be rejected and a different approach taken. It requires experience to make this judgment. Even singlet states can suffer from spin-contamination, for example the H2 dissociation curve is discontinuous
at the point when spin-contamination states (known as the Coulson-Fischer point
).
Despite this drawback, the unrestricted Hartree–Fock method is used frequently, and in preference to the restricted open-shell Hartree–Fock (ROHF) method, because UHF is simpler to code, easier to develop post-Hartree–Fock methods with, and returns unique functions unlike ROHF where different Fock operators can give the same final wave
function.
Unrestricted Hartree–Fock theory was discovered by Gaston Berthier and subsequently developed by John Pople
; it is found in almost all ab initio programs.
Open shell
In the context of atomic orbitals, an open shell is a valence shell which is not completely filled with electrons or that has not given all of its valence electrons through chemical bonds with other atoms or molecules during a chemical reaction. Atoms generally reach a noble gas configuration in a...
molecules where the number of electrons of each spin are not equal. While restricted Hartree–Fock theory uses a single molecular orbital twice, once multiplied by the α spin function and once multiplied by the β spin function in the Slater determinant
Slater determinant
In quantum mechanics, a Slater determinant is an expression that describes the wavefunction of a multi-fermionic system that satisfies anti-symmetry requirements and consequently the Pauli exclusion principle by changing sign upon exchange of fermions . It is named for its discoverer, John C...
, unrestricted Hartree–Fock theory uses different molecular orbitals for the α and β electrons. This has been called a different orbitals for different spins (DODS) method. The result is a pair of coupled Roothaan equations
Roothaan equations
The Roothaan equations are a representation of the Hartree-Fock equation in a non orthonormal basis set which can be of Gaussian-type or Slater-type. It applies to closed-shell molecules or atoms where all molecular orbitals or atomic orbitals, respectively, are doubly occupied. This is generally...
, known as the Pople–Nesbet–Berthier equations.
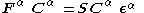
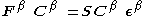
Where
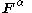 and
and 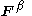 are the Fock matrices
are the Fock matricesFock matrix
In the Hartree-Fock method of quantum mechanics, the Fock matrix is a matrix approximating the single-electron energy operator of a given quantum system in a given set of basis vectors....
for the
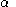 and
and 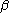 orbitals,
orbitals, 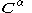 and
and 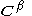 are the matrices of coefficients for the
are the matrices of coefficients for the  and
and 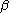 orbitals, S is the overlap matrix
orbitals, S is the overlap matrixOverlap matrix
The overlap matrix is a square matrix, used in quantum chemistry to describe the inter-relationship of a set of basis vectors of a quantum system. In particular, if the vectors are orthogonal to one another, the overlap matrix will be diagonal. In addition, if the basis vectors form an...
of the basis functions, and
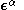 and
and 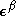 are the (diagonal, by convention) matrices of orbital energies for the
are the (diagonal, by convention) matrices of orbital energies for the 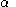 and
and 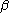 orbitals. The pair of equations are coupled because the Fock matrix elements of one spin contains coefficients of both spin as the orbital has to be optimized in the average field of all other electrons. The final result is a set of molecular orbitals and orbital energies for the α spin electrons and a set of molecular orbitals and orbital energies for the β electrons.
orbitals. The pair of equations are coupled because the Fock matrix elements of one spin contains coefficients of both spin as the orbital has to be optimized in the average field of all other electrons. The final result is a set of molecular orbitals and orbital energies for the α spin electrons and a set of molecular orbitals and orbital energies for the β electrons.This method has one drawback. A single Slater determinant
Slater determinant
In quantum mechanics, a Slater determinant is an expression that describes the wavefunction of a multi-fermionic system that satisfies anti-symmetry requirements and consequently the Pauli exclusion principle by changing sign upon exchange of fermions . It is named for its discoverer, John C...
of different orbitals for different spins is not a satisfactory eigenfunction of the total spin operator -
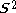 . The ground state is contaminated
. The ground state is contaminatedSpin contamination
In computational chemistry, spin contamination is the artificial mixing of different electronic spin-states. This can occur when an approximate orbital-based wave function is represented in an unrestricted form – that is, when the spatial parts of α and β spin-orbitals are permitted to differ....
by excited states. If there is one more electron of α spin than β spin, the ground state is a doublet. The average value of
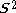 , written <
, written <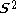 >, should be
>, should be 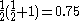 but will actually be rather more than this value as the doublet state is contaminated by a quadruplet state. A triplet state with two excess α electrons should have <
but will actually be rather more than this value as the doublet state is contaminated by a quadruplet state. A triplet state with two excess α electrons should have <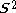 > = 1 (1 + 1) = 2, but it will be larger as the triplet is contaminated by a quintuplet state. When carrying out unrestricted Hartree–Fock calculations, it is always necessary to check this contamination. For example, with a doublet state, if <
> = 1 (1 + 1) = 2, but it will be larger as the triplet is contaminated by a quintuplet state. When carrying out unrestricted Hartree–Fock calculations, it is always necessary to check this contamination. For example, with a doublet state, if <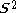 > = 0.8 or less, it is probably satisfactory. If it is 1.0 or so, it is certainly not satisfactory and the calculation should be rejected and a different approach taken. It requires experience to make this judgment. Even singlet states can suffer from spin-contamination, for example the H2 dissociation curve is discontinuous
> = 0.8 or less, it is probably satisfactory. If it is 1.0 or so, it is certainly not satisfactory and the calculation should be rejected and a different approach taken. It requires experience to make this judgment. Even singlet states can suffer from spin-contamination, for example the H2 dissociation curve is discontinuousat the point when spin-contamination states (known as the Coulson-Fischer point
Coulson-Fischer theory
In theoretical chemistry and molecular physics, Coulson-Fischer theory provides a quantum mechanical description of the electronic structure of molecules...
).
Despite this drawback, the unrestricted Hartree–Fock method is used frequently, and in preference to the restricted open-shell Hartree–Fock (ROHF) method, because UHF is simpler to code, easier to develop post-Hartree–Fock methods with, and returns unique functions unlike ROHF where different Fock operators can give the same final wave
function.
Unrestricted Hartree–Fock theory was discovered by Gaston Berthier and subsequently developed by John Pople
John Pople
Sir John Anthony Pople, KBE, FRS, was a Nobel-Prize winning theoretical chemist. Born in Burnham-on-Sea, Somerset, England, he attended Bristol Grammar School. He won a scholarship to Trinity College, Cambridge in 1943. He received his B. A. in 1946. Between 1945 and 1947 he worked at the Bristol...
; it is found in almost all ab initio programs.

