Saturation Vapor Curve
Encyclopedia
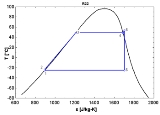
The saturation vapor curve usually states for the separation curve between the two-phase state and the vapor state in the T-s diagram. When used in a power cycle
, the fluid expansion depends strongly on the nature of this saturation curve :
Thermodynamic cycle
A thermodynamic cycle consists of a series of thermodynamic processes transferring heat and work, while varying pressure, temperature, and other state variables, eventually returning a system to its initial state...
, the fluid expansion depends strongly on the nature of this saturation curve :
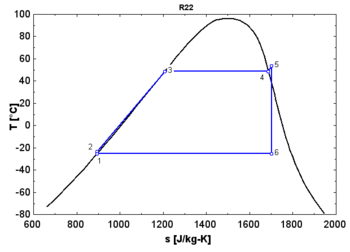
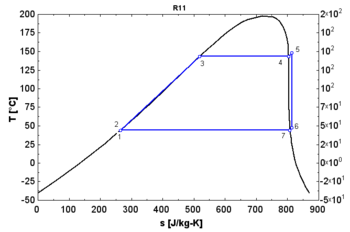
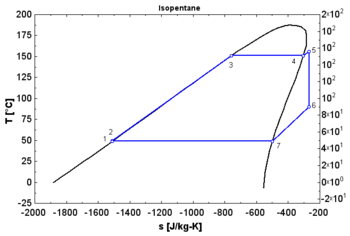
- A "wet" fluid shows a negative saturation vapor curve. If overheating before the expansion is limited, a two-phase state is obtained at the end of the expansion.

- An "isentropic" fluid shows a vertical saturation vapor curve. It remains very close to the saturated vapor state after an hypothetical isentropic expansion.

- A "dry" fluid shows a positive saturation vapor curve. It is in dry vapor state at the end of the expansion, and strongly overheated.


