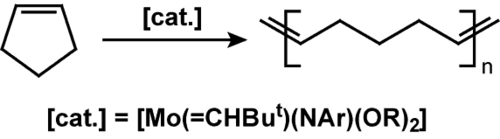
Ring opening metathesis polymerisation
Encyclopedia
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis
chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain
in cyclic olefins (e.g. norbornene
or cyclopentene
) and a wide variety of catalysts have been discovered. Research has shown that the addition of substituents to the monomer and the choice of solvent can alter the molecular weight of the polymer produced.
The ROMP reaction is catalyzed primarily through the formation of metal-carbene complexes as first reported by Nobel Prize winner Yves Chauvin
and his colleague Jean-Louis Hérisson although a hydride mechanism has also been reported. The initiation of the carbene species occurs through numerous pathways; solvent interactions, substituent interactions, and co-catalysts all can contribute to the production of the reactive catalytic species
.
The ROMP catalytic cycle requires a strained cyclic structure because the driving force of the reaction is relief of ring strain. After formation of the metal-carbene species, the carbene attacks the double bond in the ring structure forming a highly strained metallacyclobutane intermediate. The ring then opens giving the beginning of the polymer: a linear chain double bonded to the metal with a terminal double bond as well. The new carbene reacts with the double bond on the next monomer, thus propagating the reaction
Solvent effects
The choice of solvent can play a vital role in the formation of the carbene species. One example of such interactions was reported by Basset, et al. regarding RuCl3 and the effects of various alcohols on its catalytic activity. Depending upon the alcohol used, the mechanistic pathway resulted in either a reactive ruthenium-hydride species or the formation of a ruthenium-carbene. Experimental results demonstrated that by altering the solvent, the molecular weight of the polymer produced was either increased or decreased. This observation could result in increased diversity of the catalytic system enabling the production of polymers of various strengths, as polymers with higher molecular weights are typically stronger than polymers of low molecular weights. Drastic differences in the rate of the reaction were also observed, thereby supporting the conclusion that the solvent plays a role in the formation of the ruthenium-carbene.
Hamilton, et al. report that altering the solvent in metal salt-type catalytic systems can drastically change the microenvironment of the system; these changes affect the tacticity of the polymer, the cis-trans ratio, and can increase the regularity of copolymers.
The position of the substituent in the ring complex has a correlation to the poisoning effect on the catalyst. However, in cases where it is non-poisoning, it is also plays a role in determining the reactivity of the substrate. Substituents cannot be placed on the carbon with the double bond or the reaction will not take place. Slugovc, et al. tested the effect of numerous functional groups on the ROMP reaction using the ‘Super-Grubbs’ catalyst, (H2IMes)(PCy3)(Cl)2Ru=CHPh. The experimental results show that the addition of common substituents to the reaction mixture can be used to tune the molecular weight range of the polymer produced.
Depending on the catalyst, some substituents can increase the rate of reaction. Norbornene epoxides increase the rate of reaction when a ruthenium trichloride/alcohol mixture is used as the catalyst. Basset, et al. contribute this rate increase to the production of a metallooxacyclobutane complex that, upon metathetic opening, gives the active ruthenium carbene complex directly. It stands to reason that other functional groups that can react with a similar mechanistic pathway will also increase the rate of reaction.
Precision control of polymer stereo- and regiochemistry is a powerful approach for the manipulation of polymer properties. This is particularly true in the case of polyolefins where tacticity and regiochemistry can have dramatic influence on the thermal, rheological, and crystallization properties. The authors Hillmyer, Kobayashi and Pitet demonstrate the regio- and stereoselective ring-opening metathesis polymerization (ROMP) of 3-substituted cis-cyclooctenes (3RCOEs, R = methyl, ethyl, hexyl, and phenyl).
are polydicyclopentadiene
products produced in a side reaction of the polymerization of norbornene.
The ROMP process is quite useful because a regular polymer with a regular amount of double bonds is formed. The resulting product can be subjected to partial or total hydrogenation or can be functionalized into more complex compounds.
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain
Strain (materials science)
In continuum mechanics, the infinitesimal strain theory, sometimes called small deformation theory, small displacement theory, or small displacement-gradient theory, deals with infinitesimal deformations of a continuum body...
in cyclic olefins (e.g. norbornene
Norbornene
Norbornene or norbornylene or norcamphene is a bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring bridged with a methylene group in the para position...
or cyclopentene
Cyclopentene
Cyclopentene is a chemical compound with the formula 58. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes.Cyclopentene is produced industrially in large amounts...
) and a wide variety of catalysts have been discovered. Research has shown that the addition of substituents to the monomer and the choice of solvent can alter the molecular weight of the polymer produced.
Mechanism
The catalysts used in the ROMP reaction include a wide variety of metals and range from a simple RuCl3/alcohol mixture to Grubbs' catalystThe ROMP reaction is catalyzed primarily through the formation of metal-carbene complexes as first reported by Nobel Prize winner Yves Chauvin
Yves Chauvin
Yves Chauvin is a French chemist and Nobel Prize laureate. He is honorary research director at the Institut français du pétrole and a member of the French Academy of Science. Chauvin received his degree from the Lyon School of Chemistry, Physics and Electronics in 1954.He was awarded the 2005...
and his colleague Jean-Louis Hérisson although a hydride mechanism has also been reported. The initiation of the carbene species occurs through numerous pathways; solvent interactions, substituent interactions, and co-catalysts all can contribute to the production of the reactive catalytic species
.
The ROMP catalytic cycle requires a strained cyclic structure because the driving force of the reaction is relief of ring strain. After formation of the metal-carbene species, the carbene attacks the double bond in the ring structure forming a highly strained metallacyclobutane intermediate. The ring then opens giving the beginning of the polymer: a linear chain double bonded to the metal with a terminal double bond as well. The new carbene reacts with the double bond on the next monomer, thus propagating the reaction
Solvent effectsSolvent effectsIn chemistry, Solvent effects is the group of effects that a solvent has on chemical reactivity. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction.-Effects on...
The choice of solvent can play a vital role in the formation of the carbene species. One example of such interactions was reported by Basset, et al. regarding RuCl3 and the effects of various alcohols on its catalytic activity. Depending upon the alcohol used, the mechanistic pathway resulted in either a reactive ruthenium-hydride species or the formation of a ruthenium-carbene. Experimental results demonstrated that by altering the solvent, the molecular weight of the polymer produced was either increased or decreased. This observation could result in increased diversity of the catalytic system enabling the production of polymers of various strengths, as polymers with higher molecular weights are typically stronger than polymers of low molecular weights. Drastic differences in the rate of the reaction were also observed, thereby supporting the conclusion that the solvent plays a role in the formation of the ruthenium-carbene.Hamilton, et al. report that altering the solvent in metal salt-type catalytic systems can drastically change the microenvironment of the system; these changes affect the tacticity of the polymer, the cis-trans ratio, and can increase the regularity of copolymers.
Substituent Effects
As previously stated, ROMP catalysis is dependent on ring strain. Therefore, the best substrates are bi- and tri-cyclic rings; however, these reactions can lead to numerous products. The addition of substituents to the ring system can result in more complex or more functional polymer products. Unfortunately, substituents on the ring can react deleteriously with some of the most common catalysts. The first Grubbs’ catalyst is poisoned by nitrile or amine groups. Many common molybdenum or tungsten metathetical catalysts are affected by oxygenate or nitrogenous groups. Thus alternative catalysts, such as ruthenium carbene complexes that are not affected by these functional groups are being researched.The position of the substituent in the ring complex has a correlation to the poisoning effect on the catalyst. However, in cases where it is non-poisoning, it is also plays a role in determining the reactivity of the substrate. Substituents cannot be placed on the carbon with the double bond or the reaction will not take place. Slugovc, et al. tested the effect of numerous functional groups on the ROMP reaction using the ‘Super-Grubbs’ catalyst, (H2IMes)(PCy3)(Cl)2Ru=CHPh. The experimental results show that the addition of common substituents to the reaction mixture can be used to tune the molecular weight range of the polymer produced.
Depending on the catalyst, some substituents can increase the rate of reaction. Norbornene epoxides increase the rate of reaction when a ruthenium trichloride/alcohol mixture is used as the catalyst. Basset, et al. contribute this rate increase to the production of a metallooxacyclobutane complex that, upon metathetic opening, gives the active ruthenium carbene complex directly. It stands to reason that other functional groups that can react with a similar mechanistic pathway will also increase the rate of reaction.
Precision control of polymer stereo- and regiochemistry is a powerful approach for the manipulation of polymer properties. This is particularly true in the case of polyolefins where tacticity and regiochemistry can have dramatic influence on the thermal, rheological, and crystallization properties. The authors Hillmyer, Kobayashi and Pitet demonstrate the regio- and stereoselective ring-opening metathesis polymerization (ROMP) of 3-substituted cis-cyclooctenes (3RCOEs, R = methyl, ethyl, hexyl, and phenyl).
Industrial Applications
Ring-opening metathesis polymerization of cycloalkenes can produce many important petrochemicals; this is of particular importance in an industrial capacity because synthetic capabilities include linear polymers from inexpensive monomers or polymers with special properties, thus compensating for an additional expense. Some examples of polymers produced on an industrial level through ROMP catalysis are Vestenamer or trans-polyoctenamer which is the metathetical polymer of cyclooctene; Norsorex or polynorbornene is another important ROMP product on the market; Telene and MettonMetton
Metton is a runny French cheese made in Franche-Comté, mostly used as an ingredient for making the Cancoillotte. The traditional process to produce Cancoillotte with Metton is to cook it in an earthenware pot with some water or milk, then to add salt and butter .-External links :* ]]...
are polydicyclopentadiene
Polydicyclopentadiene
Polydicyclopentadiene is a polymer which is formed through ring opening metathesis polymerisation of dicyclopentadiene . The difference between the various systems lies in the type of catalyst used to create the polymer, but the final polymer properties are similar...
products produced in a side reaction of the polymerization of norbornene.
The ROMP process is quite useful because a regular polymer with a regular amount of double bonds is formed. The resulting product can be subjected to partial or total hydrogenation or can be functionalized into more complex compounds.