
Nordic Institute of Dental Materials
Encyclopedia
The Nordic Institute of Dental Materials AS (NIOM AS) is the Nordic Cooperative Body for dental biomaterials. The Institute’s activities in research, materials testing, standardisation and research-based consulting are directed towards dental health services and health authorities in the Nordic countries. The Institute is owned jointly by UniRand (a wholly owned subsidiary of the University of Oslo) and the Norwegian Ministry of Health and Care Services. Activities are financed by the Nordic Council
of Ministers and the Nordic ministries for health services. Materials testing and consulting services also generate income.
As a joint Nordic resource center, NIOM collaborates with dental schools and research institutions and provides services to government health authorities, dental professionals, and the public in the Nordic countries in the field of dental biomaterials.
. A programme for testing and certification of dental biomaterials on the Nordic market was established, and the first lists of certified materials were published in 1974. Products were tested every year, and the Nordic health authorities required, or strongly recommended, that dentists used NIOM certified products. In 1992, the laboratory obtained official accreditation for testing dental biomaterial
s. These testing and certification activities continued until 1998 when the European Medical device
s directive came into force, introducing a new, joint European regulatory scheme for the certification (CE marking
) of medical and dental biomaterials and devices.
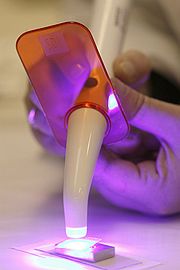 Today, the Institute maintains its competency in the accredited
Today, the Institute maintains its competency in the accredited
testing of dental biomaterials, and promotes this activity with manufacturers and regulatory bodies.
Before 2004, had the responsibility as a Notified Body
for the use of the CE mark
on dental products.
The provision of an accredited laboratory service to industry, health authorities and other parties remains one of NIOM's core activities.
On January 1 2010 NIOM’s status changed from a Nordic Institute to a Nordic Co-operative Body. This means that ownership of NIOM is transferred from the Nordic Council
of Ministers to UniRand a.s. (an arm of the University of Oslo) and the Norwegian Ministry of Health and Care Services to create a proprietary company, Nordic Institute of Dental Materials (NIOM). NIOM continues the work with the same staff and professional services.
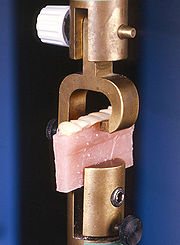 Research is a major part of the portfolio of work at NIOM. The institute collaborates with universities and research centres in dentistry, medicine and materials science in the Nordic countries and worldwide. Research underpins both standardization activities and the information and recommendations provided to health authorities, the dental profession and the public. Projects include material characterization and properties, biocompatibility
Research is a major part of the portfolio of work at NIOM. The institute collaborates with universities and research centres in dentistry, medicine and materials science in the Nordic countries and worldwide. Research underpins both standardization activities and the information and recommendations provided to health authorities, the dental profession and the public. Projects include material characterization and properties, biocompatibility
and clinical performance.
s and medical device
s to dental personnel, health authorities and to the public. This is promulgated through articles in the Nordic dental journals, journals for dental technicians, KDM-reports (Kunskapscenter för Dentala Material, http://www.socialstyrelsen.se/tandvard/dentalamaterial) and a continuous updating of the NIOM website. There is ongoing work on a register of dental products ("DMN" - Dentala Material Norden, http://dmn.bolder.se/).
NIOM also provides on-line services, E-mail and telephone, related to dental biomaterials.
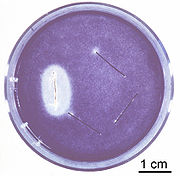 NIOM has for many years contributed to standardization at an international level both through the International organization for Standardization - ISO and within Europe through the Comité Européen de Normalisation
NIOM has for many years contributed to standardization at an international level both through the International organization for Standardization - ISO and within Europe through the Comité Européen de Normalisation
(CEN). Development of new standards and revision of existing standards have been the main focus. Standards in dentistry set requirements for the properties of dental materials and dental instrumentation, and prescribe procedures for the testing of dental materials. Participation in standardization work for dental products and for biocompatibility
in general allows NIOM to have an influence on test methods and requirements for dental products. NIOM has had, and still has, a major impact on the selection of methodology and requirements of the dental product standards, and the requirements are often based on results from research activities at NIOM.
International standardization is organized in different Technical Committees (TC), each dealing with a specific field of interest. Each committee is again divided into smaller subcommittees and working groups devoted to different topics of the field. Scientists from the institute are technical experts and conveners of working groups within the following technical committees: ISO/TC 106 Dentistry; ISO/TC 194 Biocompatibility of medical devices; CEN/TC 55 Dentistry, and CEN/TC 206 Biocompatibility of medical and dental materials and devices. Standards Norway
(SN) is the national member body in the European and International standardization organizations.
Nordic Council
The Nordic Council is a geo-political, inter-parliamentary forum for co-operation between the Nordic countries. It was established following World War II and its first concrete result was the introduction in 1952 of a common labour market and free movement across borders without passports for the...
of Ministers and the Nordic ministries for health services. Materials testing and consulting services also generate income.
As a joint Nordic resource center, NIOM collaborates with dental schools and research institutions and provides services to government health authorities, dental professionals, and the public in the Nordic countries in the field of dental biomaterials.
History
NIOM was established in 1972, as a joint Nordic institute located close to Ullevaal Stadium in OsloOslo
Oslo is a municipality, as well as the capital and most populous city in Norway. As a municipality , it was established on 1 January 1838. Founded around 1048 by King Harald III of Norway, the city was largely destroyed by fire in 1624. The city was moved under the reign of Denmark–Norway's King...
. A programme for testing and certification of dental biomaterials on the Nordic market was established, and the first lists of certified materials were published in 1974. Products were tested every year, and the Nordic health authorities required, or strongly recommended, that dentists used NIOM certified products. In 1992, the laboratory obtained official accreditation for testing dental biomaterial
Biomaterial
A biomaterial is any matter, surface, or construct that interacts with biological systems. The development of biomaterials, as a science, is about fifty years old. The study of biomaterials is called biomaterials science. It has experienced steady and strong growth over its history, with many...
s. These testing and certification activities continued until 1998 when the European Medical device
Medical device
A medical device is a product which is used for medical purposes in patients, in diagnosis, therapy or surgery . Whereas medicinal products achieve their principal action by pharmacological, metabolic or immunological means. Medical devices act by other means like physical, mechanical, thermal,...
s directive came into force, introducing a new, joint European regulatory scheme for the certification (CE marking
CE mark
CE marking is a mandatory conformity mark for products placed on the market in the European Economic Area . With the CE marking on a product the manufacturer ensures that the product conforms with the essential requirements of the applicable EC directives...
) of medical and dental biomaterials and devices.

Accreditation
Accreditation is a process in which certification of competency, authority, or credibility is presented.Organizations that issue credentials or certify third parties against official standards are themselves formally accredited by accreditation bodies ; hence they are sometimes known as "accredited...
testing of dental biomaterials, and promotes this activity with manufacturers and regulatory bodies.
Before 2004, had the responsibility as a Notified Body
Notified Body
A Notified Body, in the European Union, is an organisation that has been accredited by a Member State to assess whether a product meets certain preordained standards. Assessment can include inspection and examination of a product, its design and manufacture...
for the use of the CE mark
CE mark
CE marking is a mandatory conformity mark for products placed on the market in the European Economic Area . With the CE marking on a product the manufacturer ensures that the product conforms with the essential requirements of the applicable EC directives...
on dental products.
The provision of an accredited laboratory service to industry, health authorities and other parties remains one of NIOM's core activities.
On January 1 2010 NIOM’s status changed from a Nordic Institute to a Nordic Co-operative Body. This means that ownership of NIOM is transferred from the Nordic Council
Nordic Council
The Nordic Council is a geo-political, inter-parliamentary forum for co-operation between the Nordic countries. It was established following World War II and its first concrete result was the introduction in 1952 of a common labour market and free movement across borders without passports for the...
of Ministers to UniRand a.s. (an arm of the University of Oslo) and the Norwegian Ministry of Health and Care Services to create a proprietary company, Nordic Institute of Dental Materials (NIOM). NIOM continues the work with the same staff and professional services.
Research

Biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term may refer to specific properties of a material without specifying where or how the material is used , or to more empirical clinical success of a whole device in...
and clinical performance.
Services for dental professionals
NIOM provides evidence- and research-based information on dental biomaterialBiomaterial
A biomaterial is any matter, surface, or construct that interacts with biological systems. The development of biomaterials, as a science, is about fifty years old. The study of biomaterials is called biomaterials science. It has experienced steady and strong growth over its history, with many...
s and medical device
Medical device
A medical device is a product which is used for medical purposes in patients, in diagnosis, therapy or surgery . Whereas medicinal products achieve their principal action by pharmacological, metabolic or immunological means. Medical devices act by other means like physical, mechanical, thermal,...
s to dental personnel, health authorities and to the public. This is promulgated through articles in the Nordic dental journals, journals for dental technicians, KDM-reports (Kunskapscenter för Dentala Material, http://www.socialstyrelsen.se/tandvard/dentalamaterial) and a continuous updating of the NIOM website. There is ongoing work on a register of dental products ("DMN" - Dentala Material Norden, http://dmn.bolder.se/).
NIOM also provides on-line services, E-mail and telephone, related to dental biomaterials.
Testing and consulting services
NIOM carries out testing according to relevant product standards and according to selected methods as requested by the client. Third party audits secure the highest quality of this service. NIOM offers consulting services in questions related to dental biomaterials. Every enquiry and assignment is treated as strictly confidential. Consultancies cover questions related to properties, performance, safety and development of dental biomaterials.Standardization

European Committee for Standardization
The European Committee for Standardization or Comité Européen de Normalisation , is a non-profit organisation whose mission is to foster the European economy in global trading, the welfare of European citizens and the environment by providing an efficient infrastructure to interested parties for...
(CEN). Development of new standards and revision of existing standards have been the main focus. Standards in dentistry set requirements for the properties of dental materials and dental instrumentation, and prescribe procedures for the testing of dental materials. Participation in standardization work for dental products and for biocompatibility
Biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term may refer to specific properties of a material without specifying where or how the material is used , or to more empirical clinical success of a whole device in...
in general allows NIOM to have an influence on test methods and requirements for dental products. NIOM has had, and still has, a major impact on the selection of methodology and requirements of the dental product standards, and the requirements are often based on results from research activities at NIOM.
International standardization is organized in different Technical Committees (TC), each dealing with a specific field of interest. Each committee is again divided into smaller subcommittees and working groups devoted to different topics of the field. Scientists from the institute are technical experts and conveners of working groups within the following technical committees: ISO/TC 106 Dentistry; ISO/TC 194 Biocompatibility of medical devices; CEN/TC 55 Dentistry, and CEN/TC 206 Biocompatibility of medical and dental materials and devices. Standards Norway
Standards Norway
Standards Norway is the main standards organization of Norway. It claims responsibility for all standardization areas except for electrotechnical and telecommunication issues. Standards Norway represents the country of Norway in CEN and ISO. Its headquarters are located at Lysaker....
(SN) is the national member body in the European and International standardization organizations.
External links
- NIOM's home page
- EU: Medical Devices - Consumer Affairs
- Norway: Directorate of Health
- Sweden: Medicinal Products Agency - Medical Devices
- Swedish National Board of Health and Welfare - Dental Materials
- Denmark: Medical Devices
- Finland: National Supervisory Authority for Welfare and Health - Medical Devices
- Iceland: Directorate of Health

