Möbius aromaticity
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, Möbius aromaticity is a special type of aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
believed to exist in a number of organic molecules. In terms of MO theory these compounds have in common a monocyclic array of molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s in which there is an odd number of out-of-phase overlaps which reveals the opposite pattern of aromatic character to Hückel systems
Hückel's rule
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931...
. The spatial configuration of the orbitals is reminiscent of a Möbius strip
Möbius strip
The Möbius strip or Möbius band is a surface with only one side and only one boundary component. The Möbius strip has the mathematical property of being non-orientable. It can be realized as a ruled surface...
, hence the name. The smallest member of this class of compounds would be trans-benzene. Möbius Systems
Möbius–Hückel concept
The Möbius-Hückel treatment is one of two predicting reaction allowedness versus forbiddeness. The concept is the counterpart of the Woodward-Hoffmannapproach...
were considered in 1964 by Edgar Heilbronner
Edgar Heilbronner
Edgar Heilbronner was a Swiss German chemist. In 1964 he published the concept of Möbius cyclic annulenes, but the first Möbius aromatic was not synthesized until 2003....
by application of the Hückel method
Hückel method
The Hückel method or Hückel molecular orbital method proposed by Erich Hückel in 1930, is a very simple linear combination of atomic orbitals molecular orbitals method for the determination of energies of molecular orbitals of pi electrons in conjugated hydrocarbon systems, such as ethene,...
but the first practical compound was synthesized in 2003 by the group of Rainer Herges.
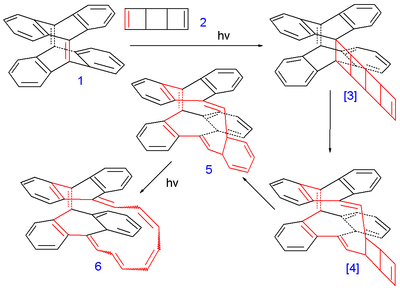
The compound (6 in the image below) was synthesized in several photochemical cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
reactions from tetradehydrodianthracene 1 and the ladderane
Ladderane
A ladderane is an organic molecule containing two or more fused rings of cyclobutane. The name is a portmanteau because the serial cyclobutane rings look like a ladder and are singly bonded like alkanes. The chemical formula for a ladderane with n rings is C2n+2H2n+6...
syn-tricyclooctadiene 2 as a substitute for cyclooctatetraene
Cyclooctatetraene
1,3,5,7-Cyclooctatetraene is an unsaturated derivative of cyclooctane, with the formula C8H8. It is also known as [8]annulene. This polyunsaturated hydrocarbon is a colorless to light yellow flammable liquid at room temperature...
.
Intermediate 5 was a mixture of 2 isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s and the final product 6 a mixture of 5 isomers with different cis and trans configurations. One of them was found to have a C2 molecular symmetry
Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
corresponding to a Möbius aromatic and another Hückel isomer was found with Cs symmetry. Despite having 16 electrons in its pi system (making it a 4n antiaromatic compound) the Heilbronner prediction
Möbius–Hückel concept
The Möbius-Hückel treatment is one of two predicting reaction allowedness versus forbiddeness. The concept is the counterpart of the Woodward-Hoffmannapproach...
was borne out because according to Herges the Möbius compound was found to have aromatic properties. With bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
s deduced from X-ray crystallography
X-ray crystallography
X-ray crystallography is a method of determining the arrangement of atoms within a crystal, in which a beam of X-rays strikes a crystal and causes the beam of light to spread into many specific directions. From the angles and intensities of these diffracted beams, a crystallographer can produce a...
a HOMA value was obtained of 0.50 (for the polyene
Polyene
Polyenes are poly-unsaturated organic compounds that contain one or more sequences of alternating double and single carbon-carbon bonds. These double carbon-carbon bonds interact in a process known as conjugation, which results in an overall lower energy state of the molecule.Organic compounds with...
part alone) and 0.35 for the whole compound which qualifies it as a moderate aromat.
It was pointed out by Henry Rzepa
Henry Rzepa
Henry S. Rzepa is a contemporary computational organic chemist. He was born in London in 1950, was educated at Wandsworth Comprehensive School, and then entered the chemistry department at Imperial College London where he graduated in 1971. Following a Ph.D...
that the conversion of intermediate 5 to 6 can proceed by either a Hückel or a Möbius transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
.
The difference was demonstrated in a hypothetical pericyclic
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
ring opening reaction to cyclododecahexaene
Cyclododecahexaene
Cyclododecahexaene or [12]annulene is a member of a series of annulenes with some interest in organic chemistry with regard to the study of aromaticity These compounds are in general unstable due to their antiaromaticity and built-up of steric strain...
. The Hückel TS (left) involves 6 electrons (arrow pushing in red) with Cs molecular symmetry
Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
conserved throughout the reaction. The ring opening is disrotatory
Disrotatory
In a conrotatory mode of an electrocyclic reaction the substituents located at the termini of a conjugated double bond system move in the same direction during ring opening or ring closure...
and suprafacial and both bond length
Bond length
- Explanation :Bond length is related to bond order, when more electrons participate in bond formation the bond will get shorter. Bond length is also inversely related to bond strength and the bond dissociation energy, as a stronger bond will be shorter...
alternation and NICS values indicate that the 6 membered ring is aromatic. The Möbius TS with 8 electrons on the other hand has lower computed activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
and is characterized by C2 symmetry, a conrotatory and antarafacial ring opening and 8-membered ring aromaticity.
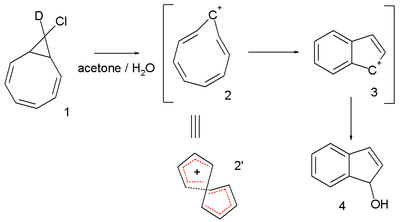
Another interesting system is the cyclononatetraenyl cation explored for over 30 years by Paul v. R. Schleyer et al. This reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
is implied in the solvolysis
Solvolysis
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
of the bicyclic chloride
Organochloride
An organochloride, organochlorine, chlorocarbon, chlorinated hydrocarbon, or chlorinated solvent is an organic compound containing at least one covalently bonded chlorine atom. Their wide structural variety and divergent chemical properties lead to a broad range of applications...
9-deutero-9'-chlorobicyclo[6.1.0]-nonatriene 1 to the indene
Indene
Indene is a flammable polycyclic hydrocarbon with chemical formula C9H8. It is composed of a benzene ring fused with a cyclopentene ring. This aromatic liquid is colorless although samples often are pale yellow...
dihydroindenol 4. The starting chloride is deuterated in only one position but in the final product deuterium is distributed at every available position. This observation is explained by invoking a twisted 8-electron cyclononatetraenyl cation 2 for which a NICS value of -13.4 (outsmarting benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
) is calculated.
In 2005 the same v. R. Schleyer questioned the 2003 Herges claim: he analyzed the same crystallographic data and concluded that there was indeed a large degree of bond length alternation resulting in a HOMA value of -0.02, a computed NICS value of -3.4 ppm also did not point towards aromaticity and (also inferred from a computer model) steric strain would prevent effective pi-orbital overlap.
A Hückel-Möbius aromaticity
Möbius–Hückel concept
The Möbius-Hückel treatment is one of two predicting reaction allowedness versus forbiddeness. The concept is the counterpart of the Woodward-Hoffmannapproach...
switch (2007) has been described based on a 28 pi-electron porphyrin
Porphyrin
Porphyrins are a group of organic compounds, many naturally occurring. One of the best-known porphyrins is heme, the pigment in red blood cells; heme is a cofactor of the protein hemoglobin. Porphyrins are heterocyclic macrocycles composed of four modified pyrrole subunits interconnected at...
system :
The phenylene
Phenylene
The phenylene group is based on a di-substituted benzene ring . For example, poly is a polymer built up from para-phenylene repeating units....
rings in this molecule are free to rotate forming a set of conformers: one with a Möbius half-twist and another with a Hückel double-twist (a figure-eight configuration) of roughly equal energy.