
Molar concentration
Encyclopedia
In chemistry, the molar concentration, 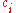 is defined as the amount
is defined as the amount
of a constituent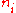 divided by the volume
divided by the volume
of the mixture :
:
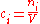
It is also called molarity, amount-of-substance concentration, amount concentration, substance concentration, or simply concentration. The volume in the definition
in the definition 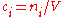 refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.
refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.
is mol/m3. However, more commonly the unit mol/L
is used.
A solution of concentration 1 mol/L is also denoted as "1 molar" (1 M).
An SI prefix
is often used to denote concentrations. Commonly used units are listed in the table below:
 is given by:
is given by:
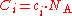
where is the Avogadro constant, approximately 6.022 mol
is the Avogadro constant, approximately 6.022 mol
−1.
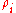 is given by:
is given by:
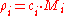
where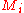 is the molar mass
is the molar mass
of constituent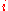 .
.
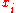 is given by:
is given by:
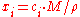
where is the average molar mass of the solution and
is the average molar mass of the solution and 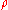 is the density
is the density
of the solution.
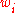 is given by:
is given by:
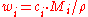
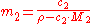
where the solute is assigned the subscript 2.
For solutions with more than one solute, the conversion is:
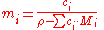
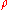 (NaCl) will be:
(NaCl) will be:
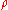 (NaCl) = 11.6 g / (11.6 g + 100 g) = 0.104 g/g = 10.4 %
(NaCl) = 11.6 g / (11.6 g + 100 g) = 0.104 g/g = 10.4 %
The density of such a solution is 1.07 g/mL, thus its volume will be:
 = (11.6 g + 100 g) / (1.07 g/mL) = 104.3 mL
= (11.6 g + 100 g) / (1.07 g/mL) = 104.3 mL
The molar concentration of NaCl in the solution is therefore:
 (NaCl) = 11.6 g / (58 g/mol * 104.3 mL) = 0.00192 mol/mL = 1.92 mol/L
(NaCl) = 11.6 g / (58 g/mol * 104.3 mL) = 0.00192 mol/mL = 1.92 mol/L
Here, 58 g/mol is the molar mass of NaCl.
Example 2: Another typical task in chemistry is the preparation of 100 mL (= 0.1 L) of a 2 mol/L solution of NaCl in water. The mass of salt needed is:
 (NaCl) = 2 mol/L * 0.1 L * 58 g/mol = 11.6 g
(NaCl) = 2 mol/L * 0.1 L * 58 g/mol = 11.6 g
To create the solution, 11.6 g NaCl are placed in a volumetric flask
, dissolved in some water, then followed by the addition of more water until the total volume reaches 100 mL.
Example 3: The density of water
is approximately 1000 g/L and its molar mass is 18.02 g/mol. Therefore, the molar concentration of water is:
 (H2O) = 1000 g/L / (18.02 g/mol) = 55.5 mol/L
(H2O) = 1000 g/L / (18.02 g/mol) = 55.5 mol/L
Likewise, the concentration of solid hydrogen
(molar mass = 2.02 g/mol) is:
 (H2) = 88 g/L / (2.02 g/mol) = 43.7 mol/L
(H2) = 88 g/L / (2.02 g/mol) = 43.7 mol/L
The concentration of pure osmium tetroxide (molar mass = 254.23 g/mol) is:
 (OsO4) = 5.1 kg/L / (254.23 g/mol) = 20.1 mol/L.
(OsO4) = 5.1 kg/L / (254.23 g/mol) = 20.1 mol/L.
Example 4: Proteins in bacteria
, such as E. coli, usually occur at about 60 copies, and the volume of a bacterium is about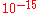 L. Thus, the number concentration
L. Thus, the number concentration  is:
is:
 = 60 / (10−15 L)= 6 L−1
= 60 / (10−15 L)= 6 L−1
The molar concentration is:
 = 6 L−1 / (6 mol−1) = 10−7 mol/L = 100 nmol/L
= 6 L−1 / (6 mol−1) = 10−7 mol/L = 100 nmol/L
 If the concentration refers to original chemical formula in solution, the molar concentration is sometimes called formal concentration. For example, if a sodium carbonate solution has a formal concentration of
If the concentration refers to original chemical formula in solution, the molar concentration is sometimes called formal concentration. For example, if a sodium carbonate solution has a formal concentration of  (Na2CO3) = 1 mol/L, the molar concentrations are
(Na2CO3) = 1 mol/L, the molar concentrations are  (Na+) = 2 mol/L and
(Na+) = 2 mol/L and  (CO32-) = 1 mol/L because the salt dissociates into these ions.
(CO32-) = 1 mol/L because the salt dissociates into these ions.
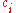 is defined as the amount
is defined as the amountAmount of substance
Amount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
of a constituent
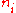 divided by the volume
divided by the volumeVolume
Volume is the quantity of three-dimensional space enclosed by some closed boundary, for example, the space that a substance or shape occupies or contains....
of the mixture
 :
: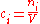
It is also called molarity, amount-of-substance concentration, amount concentration, substance concentration, or simply concentration. The volume
 in the definition
in the definition 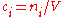 refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.
refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.Units
The SI-unitInternational System of Units
The International System of Units is the modern form of the metric system and is generally a system of units of measurement devised around seven base units and the convenience of the number ten. The older metric system included several groups of units...
is mol/m3. However, more commonly the unit mol/L
Litre
pic|200px|right|thumb|One litre is equivalent to this cubeEach side is 10 cm1 litre water = 1 kilogram water The litre is a metric system unit of volume equal to 1 cubic decimetre , to 1,000 cubic centimetres , and to 1/1,000 cubic metre...
is used.
A solution of concentration 1 mol/L is also denoted as "1 molar" (1 M).
- 1 mol/L = 1 mol/dmDecimetreA decimetre is a unit of length in the metric system, equal to one tenth of a metre, the SI base unit of length. In simple words there are 10 cm in a decimetre....
3 = 1 mol dm−3 = 1 M = 1000 mol/m3.
An SI prefix
SI prefix
The International System of Units specifies a set of unit prefixes known as SI prefixes or metric prefixes. An SI prefix is a name that precedes a basic unit of measure to indicate a decadic multiple or fraction of the unit. Each prefix has a unique symbol that is prepended to the unit symbol...
is often used to denote concentrations. Commonly used units are listed in the table below:
| Name | Abbreviation | Concentration | Concentration (SI-unit) |
|---|---|---|---|
| millimolar | mM | 10−3 mol/dm3 | 100 mol/m3 |
| micromolar | μM | 10−6 mol/dm3 | 10−3 mol/m3 |
| nanomolar | nM | 10−9 mol/dm3 | 10−6 mol/m3 |
| picomolar | pM | 10−12 mol/dm3 | 10−9 mol/m3 |
| femtomolar | fM | 10−15 mol/dm3 | 10−12 mol/m3 |
| attomolar | aM | 10−18 mol/dm3 | 10−15 mol/m3 |
| zeptomolar | zM | 10−21 mol/dm3 | 10−18 mol/m3 |
| yoctomolar | yM | 10−24 mol/dm3 (1 molecule per 1.6 L) |
10−21 mol/m3 |
Number concentration
The conversion to number concentration is given by:
is given by: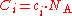
where
 is the Avogadro constant, approximately 6.022 mol
is the Avogadro constant, approximately 6.022 molMole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
−1.
Mass concentration
The conversion to mass concentration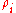 is given by:
is given by: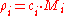
where
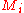 is the molar mass
is the molar massMolar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
of constituent
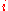 .
.Mole fraction
The conversion to mole fraction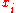 is given by:
is given by: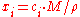
where
 is the average molar mass of the solution and
is the average molar mass of the solution and 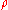 is the density
is the densityDensity
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
of the solution.
Mass fraction
The conversion to mass fraction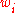 is given by:
is given by: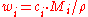
Molality
The conversion to molality (for binary mixtures) is: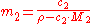
where the solute is assigned the subscript 2.
For solutions with more than one solute, the conversion is:
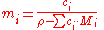
Dependence on volume
Molar concentration depends on the variation of the volume of the solution due mainly to thermal expansion.Examples
Example 1: Consider 11.6 g of NaCl dissolved in 100 g of water. The final mass concentration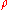 (NaCl) will be:
(NaCl) will be: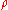 (NaCl) = 11.6 g / (11.6 g + 100 g) = 0.104 g/g = 10.4 %
(NaCl) = 11.6 g / (11.6 g + 100 g) = 0.104 g/g = 10.4 %The density of such a solution is 1.07 g/mL, thus its volume will be:
 = (11.6 g + 100 g) / (1.07 g/mL) = 104.3 mL
= (11.6 g + 100 g) / (1.07 g/mL) = 104.3 mLThe molar concentration of NaCl in the solution is therefore:
 (NaCl) = 11.6 g / (58 g/mol * 104.3 mL) = 0.00192 mol/mL = 1.92 mol/L
(NaCl) = 11.6 g / (58 g/mol * 104.3 mL) = 0.00192 mol/mL = 1.92 mol/LHere, 58 g/mol is the molar mass of NaCl.
Example 2: Another typical task in chemistry is the preparation of 100 mL (= 0.1 L) of a 2 mol/L solution of NaCl in water. The mass of salt needed is:
 (NaCl) = 2 mol/L * 0.1 L * 58 g/mol = 11.6 g
(NaCl) = 2 mol/L * 0.1 L * 58 g/mol = 11.6 gTo create the solution, 11.6 g NaCl are placed in a volumetric flask
Volumetric flask
A volumetric flask is a piece of laboratory glassware, a type of laboratory flask, used in analytical chemistry for the preparation of solutions. It is made of glass or plastic and consists of a flat bottomed bulb with a long neck, usually fitted with a stopper. The stopper is normally made in a...
, dissolved in some water, then followed by the addition of more water until the total volume reaches 100 mL.
Example 3: The density of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
is approximately 1000 g/L and its molar mass is 18.02 g/mol. Therefore, the molar concentration of water is:
 (H2O) = 1000 g/L / (18.02 g/mol) = 55.5 mol/L
(H2O) = 1000 g/L / (18.02 g/mol) = 55.5 mol/LLikewise, the concentration of solid hydrogen
Solid hydrogen
Solid hydrogen is the solid state of the element hydrogen, achieved by decreasing the temperature below hydrogen's melting point of 14.01 K . It was collected for the first time by James Dewar in 1899 and published with the title "Sur la solidification de l'hydrogène" in the Annales de Chimie et...
(molar mass = 2.02 g/mol) is:
 (H2) = 88 g/L / (2.02 g/mol) = 43.7 mol/L
(H2) = 88 g/L / (2.02 g/mol) = 43.7 mol/LThe concentration of pure osmium tetroxide (molar mass = 254.23 g/mol) is:
 (OsO4) = 5.1 kg/L / (254.23 g/mol) = 20.1 mol/L.
(OsO4) = 5.1 kg/L / (254.23 g/mol) = 20.1 mol/L.Example 4: Proteins in bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
, such as E. coli, usually occur at about 60 copies, and the volume of a bacterium is about
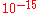 L. Thus, the number concentration
L. Thus, the number concentration  is:
is: = 60 / (10−15 L)= 6 L−1
= 60 / (10−15 L)= 6 L−1The molar concentration is:
 = 6 L−1 / (6 mol−1) = 10−7 mol/L = 100 nmol/L
= 6 L−1 / (6 mol−1) = 10−7 mol/L = 100 nmol/L
 (Na2CO3) = 1 mol/L, the molar concentrations are
(Na2CO3) = 1 mol/L, the molar concentrations are  (Na+) = 2 mol/L and
(Na+) = 2 mol/L and  (CO32-) = 1 mol/L because the salt dissociates into these ions.
(CO32-) = 1 mol/L because the salt dissociates into these ions.
