
Mass-action ratio
Encyclopedia
The mass-action ratio, often denoted by 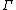 , is the ratio of product concentrations to reactant concentrations.
, is the ratio of product concentrations to reactant concentrations.
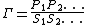
If the product and reactant concentrations are at equilibrium then the mass-action ratio will equal the equilibrium constant. At equilibrium:
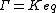
The ratio of the mass-action ratio to the equilibrium constant is often called the disequilibrium ratio, denoted by the symbol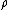 .
.
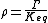
At equilibrium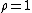 . When the reaction is out of equilibrium
. When the reaction is out of equilibrium 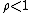 but always greater than zero.
but always greater than zero.
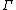 , is the ratio of product concentrations to reactant concentrations.
, is the ratio of product concentrations to reactant concentrations.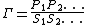
If the product and reactant concentrations are at equilibrium then the mass-action ratio will equal the equilibrium constant. At equilibrium:
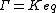
The ratio of the mass-action ratio to the equilibrium constant is often called the disequilibrium ratio, denoted by the symbol
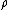 .
.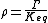
At equilibrium
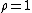 . When the reaction is out of equilibrium
. When the reaction is out of equilibrium 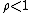 but always greater than zero.
but always greater than zero.Other sources
- Atkins, P.W.Peter AtkinsPeter William Atkins is a British chemist and former Professor of Chemistry at the University of Oxford and a Fellow of Lincoln College. He is a prolific writer of popular chemistry textbooks, including Physical Chemistry, Inorganic Chemistry, and Molecular Quantum Mechanics...
(1978). Physical Chemistry Oxford University PressOxford University PressOxford University Press is the largest university press in the world. It is a department of the University of Oxford and is governed by a group of 15 academics appointed by the Vice-Chancellor known as the Delegates of the Press. They are headed by the Secretary to the Delegates, who serves as...
ISBN 0-7167-3539-X - Trevor Palmer (2001) Enzymes: biochemistry, biotechnology and clinical chemistry Chichester Horwood Publishing ISBN 1-8985-6378-0

