
ECTD
Encyclopedia
The electronic Common Technical Document (eCTD) is an interface for the pharmaceutical industry to agency transfer of regulatory information.
The content is based on the Common Technical Document
(CTD) format.
It was developed by the International Conference on Harmonisation (ICH) Multidisciplinary Group 2 Expert Working Group (ICH M2 EWG). As of January 1, 2008, the U.S. Food and Drug Administration announced that the eCTD is the preferred format for electronic submission
s. To date, over 98,000 eCTD sequences have been submitted to the FDA. Although the agency has not released an expected target date, the FDA revealed during the 2009 DIA Annual Meeting that it is looking at draft legislation to require eCTD.
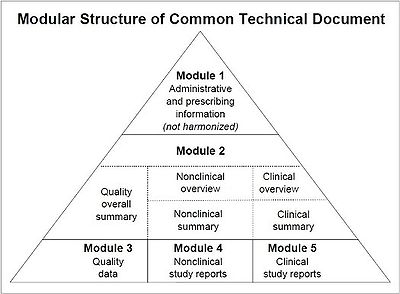 The eCTD has five modules
The eCTD has five modules
A full table of contents could be quite large.
There are two categories of modules:
The CTD only defines the content of the common modules. The contents of the Regional Module 1 are defined by each of the ICH regions (USA, Europe and Japan).
Each submission message constitutes one "sequence". A cumulative eCTD consists of one or more sequences. While a single sequence may be viewed with web browser and the ICH stylesheet provided, viewing a cumulative eCTD requires specialized eCTD viewers.
The top part of the directory structure is as follows:
The string ctd-123456/0000 is just an example.
For example:
The purpose of this file is twofold:
They must be placed in the directory:
See entry 377 in Appendix 4.
See entry 371 to 376 in Appendix 4.
They must follow a naming convention.
The DTD of the backbone is in Appendix 8.
It must be place in the above directory.
Industry <-----> Message <-----> Agency
The lifecycle management is composed at least of:
The content is based on the Common Technical Document
Common Technical Document
The Common Technical Document is a set of specification for application dossier for the registration of Medicines and designed to be used across Europe, Japan and the United States. It was developed by the European Medicines Agency , the Food and Drug Administration and the Ministry of Health,...
(CTD) format.
It was developed by the International Conference on Harmonisation (ICH) Multidisciplinary Group 2 Expert Working Group (ICH M2 EWG). As of January 1, 2008, the U.S. Food and Drug Administration announced that the eCTD is the preferred format for electronic submission
Electronic submission
An electronic submission refers to a manuscript submitted by electronic means: that is, via e-mail or a web form on the Internet, or on an electronic medium such as a compact disc, a hard disk or a USB flash drive. Traditionally, a manuscript referred to anything that was explicitly "written by hand"...
s. To date, over 98,000 eCTD sequences have been submitted to the FDA. Although the agency has not released an expected target date, the FDA revealed during the 2009 DIA Annual Meeting that it is looking at draft legislation to require eCTD.
Pharmaceutical point of view

- 1 Administrative Information and Prescribing Information
- 2 Common Technical Document Summaries
- 3 Quality
- 4 Nonclinical Study Reports
- 5 Clinical Study Reports
A full table of contents could be quite large.
There are two categories of modules:
- Regional module: 1 (different for each region; i.e., country)
- Common modules: 2-5 (common to all the regions)
The CTD only defines the content of the common modules. The contents of the Regional Module 1 are defined by each of the ICH regions (USA, Europe and Japan).
eCTD (data structure)
The eCTD is a message specification for the transfer of files and metadata from a submitter to a receiver. The primary technical components are:- A high level folder structure (required)
- An XML "backbone" file which provides metadata about content files and lifecycle instructions for the receiving system
- An optional lower level folder structure (recommended folder names are provided in Appendix 4 of the eCTD specification)
- Associated Document Type Definitions (DTDs) and stylesheets.
Each submission message constitutes one "sequence". A cumulative eCTD consists of one or more sequences. While a single sequence may be viewed with web browser and the ICH stylesheet provided, viewing a cumulative eCTD requires specialized eCTD viewers.
The top part of the directory structure is as follows:
ctd-123456/0000/index.xml
ctd-123456/0000/index-md5.txt
ctd-123456/0000/m1
ctd-123456/0000/m2
ctd-123456/0000/m3
ctd-123456/0000/m4
ctd-123456/0000/m5
ctd-123456/0000/util
The string ctd-123456/0000 is just an example.
Backbone (header)
This is the fileindex.xml in the submission sequence number folder.For example:
ctd-123456/0000/index.xml
The purpose of this file is twofold:
- Manage meta-data for the entire submission
- Constitute a comprehensive table of contents and provide corresponding navigation aid.
Stylesheets
Stylesheets should be included that support the presentation and navigation.They must be placed in the directory:
ctd-123456/0000/util/style
See entry 377 in Appendix 4.
DTDs
DTDs must be placed in the directory:
ctd-123456/0000/util/dtd
See entry 371 to 376 in Appendix 4.
They must follow a naming convention.
The DTD of the backbone is in Appendix 8.
It must be place in the above directory.
Business process (protocol)
The business process to be supported can be described as follows:Industry <-----> Message <-----> Agency
The lifecycle management is composed at least of:
- Initial submission: should be self-contained.
- Incremental updates: with its sequence number.
See also
- Clinical trialClinical trialClinical trials are a set of procedures in medical research and drug development that are conducted to allow safety and efficacy data to be collected for health interventions...
- Clinical Data Interchange Standards ConsortiumClinical Data Interchange Standards ConsortiumClinical Data Interchange Standards Consortium is a non-profit organization, whose mission is "to develop and support global, platform-independent data standards that enable information system interoperability to improve medical research and related areas of health-care". Their main project, the...
- European Medicines AgencyEuropean Medicines AgencyThe European Medicines Agency is a European agency for the evaluation of medicinal products. From 1995 to 2004, the European Medicines Agency was known as European Agency for the Evaluation of Medicinal Products.Roughly parallel to the U.S...
(EMA) - Food and Drug AdministrationFood and Drug AdministrationThe Food and Drug Administration is an agency of the United States Department of Health and Human Services, one of the United States federal executive departments...
(FDA) - Ministry of Health, Labour and Welfare (Japan).
External links
- ICH eCTD Specification V 3.2.2 (The specification)
- ICH M2 ESTRI Main
- Electronic Common Technical Document (eCTD) (FDA)
- EUDRALEX Volume 2 - Pharmaceutical Legislation : Notice to Applicants (EU legislation, contains section on eCTD)
- IT Pharma Validation Europe (Organization: CSV Validation Network)
- Exalon eCTD News - free and strictly neutral eCTD related news from regulators
- The eCTD summit blog
- eCTDBlog.com
- Regional file format requirements for eCTD

