
Chemi-ionization
Encyclopedia
Chemi-ionization is the formation of an ion
through the reaction of a gas phase atom
or molecule
with an atom or molecule in an excited state
.
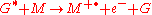
where G is the excited state species (indicated by the superscripted asterisk), and M is the species that is ionized by the loss of an electron
to form the radical
cation (indicated by the superscripted "plus-dot").
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
through the reaction of a gas phase atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
or molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
with an atom or molecule in an excited state
Excited state
Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
.
Reactions
Chemi-ionization can be represented by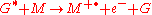
where G is the excited state species (indicated by the superscripted asterisk), and M is the species that is ionized by the loss of an electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
to form the radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
cation (indicated by the superscripted "plus-dot").

